Response of BALB/c mice to a monovalent influenza A (H1N1) 2009 split vaccine
- PMID: 20118968
- PMCID: PMC4076736
- DOI: 10.1038/cmi.2009.116
Response of BALB/c mice to a monovalent influenza A (H1N1) 2009 split vaccine
Abstract
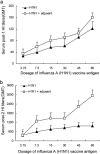
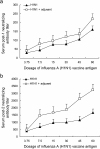
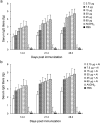
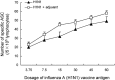
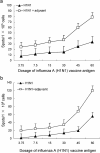
The novel influenza A (H1N1) 2009 virus has emerged to cause the first pandemic of the twenty-first century. Disease outbreaks caused by the influenza A (H1N1) virus have prompted concerns about the potential for a pandemic and have driven the development of vaccines against this subtype of influenza A. In this study, we developed a monovalent influenza A (H1N1) split vaccine and evaluated its effects in BALB/c mice. Mice were immunized subcutaneously with 2 doses of the vaccine containing hemagglutinin (HA) alone or HA plus an aluminum hydroxide (Al(OH)(3)) adjuvant. Immunization with varying doses of HA (3.75, 7.5, 15, 30, 45 or 60 microg) was performed to induce the production of neutralizing antibodies. The vaccine elicited strong hemagglutination inhibition (HI) and microneutralization, and addition of the adjuvant augmented the antibody response. A preliminary safety evaluation showed that the vaccine was not toxic at large doses (0.5 ml containing 60 microg HA+600 microg Al(OH)(3) or 60 microg HA). Moreover, the vaccine was found to be safe at a dose of 120 microg HA+1200 microg Al(OH)(3) or 120 microg HA in 1.0 ml in rats. In conclusion, the present study provides support for the clinical evaluation of influenza A (H1N1) vaccination as a public health intervention to mitigate a possible pandemic. Additionally, our findings support the further evaluation of the vaccine used in this study in primates or humans.
Figures

Similar articles
-
[Immune protective effectiveness of seasonal influenza spilt vaccine against homologous and heterogonous subtypes of influenza virus in mice].Bing Du Xue Bao. 2011 May;27(3):265-73. Bing Du Xue Bao. 2011. PMID: 21774253 Chinese.
-
AF03-adjuvanted and non-adjuvanted pandemic influenza A (H1N1) 2009 vaccines induce strong antibody responses in seasonal influenza vaccine-primed and unprimed mice.Vaccine. 2010 Apr 19;28(18):3076-9. doi: 10.1016/j.vaccine.2010.02.050. Epub 2010 Mar 1. Vaccine. 2010. PMID: 20193791
-
Evaluating the Immune Response of Recombinant H1N1 Hemagglutinin with MF59 Adjuvant in Animal Model as a Novel Alternative to the Influenza Vaccine.Iran J Allergy Asthma Immunol. 2020 Oct 18;19(5):497-508. doi: 10.18502/ijaai.v19i5.4465. Iran J Allergy Asthma Immunol. 2020. PMID: 33463117
-
Randomized, multicenter trial of a single dose of AS03-adjuvanted or unadjuvanted H1N1 2009 pandemic influenza vaccine in children 6 months to <9 years of age: safety and immunogenicity.Pediatr Infect Dis J. 2012 Aug;31(8):848-58. doi: 10.1097/INF.0b013e31825e6cd6. Pediatr Infect Dis J. 2012. PMID: 22801094 Clinical Trial.
-
[Tetravaccine--new fundamental approach to prevention of influenza pandemic].Zh Mikrobiol Epidemiol Immunobiol. 2007 Jul-Aug;(4):15-9. Zh Mikrobiol Epidemiol Immunobiol. 2007. PMID: 17882832 Review. Russian.
Cited by
-
Immunization with a live attenuated H7N9 influenza vaccine protects mice against lethal challenge.PLoS One. 2015 Apr 17;10(4):e0123659. doi: 10.1371/journal.pone.0123659. eCollection 2015. PLoS One. 2015. PMID: 25884801 Free PMC article.
-
Effective Suckling C57BL/6, Kunming, and BALB/c Mouse Models with Remarkable Neurological Manifestation for Zika Virus Infection.Viruses. 2017 Jun 29;9(7):165. doi: 10.3390/v9070165. Viruses. 2017. PMID: 28661429 Free PMC article.
-
Virus-like particle vaccine containing hemagglutinin confers protection against 2009 H1N1 pandemic influenza.Clin Vaccine Immunol. 2011 Dec;18(12):2010-7. doi: 10.1128/CVI.05206-11. Epub 2011 Oct 26. Clin Vaccine Immunol. 2011. PMID: 22030367 Free PMC article.
-
Multiple-clade H5N1 influenza split vaccine elicits broad cross protection against lethal influenza virus challenge in mice by intranasal vaccination.PLoS One. 2012;7(1):e30252. doi: 10.1371/journal.pone.0030252. Epub 2012 Jan 18. PLoS One. 2012. PMID: 22279575 Free PMC article.
-
Characterization of the 2009 pandemic A/Beijing/501/2009 H1N1 influenza strain in human airway epithelial cells and ferrets.PLoS One. 2012;7(9):e46184. doi: 10.1371/journal.pone.0046184. Epub 2012 Sep 26. PLoS One. 2012. PMID: 23049974 Free PMC article.
References
-
- Smith GJ, Vijaykrishna D, Bahl J, Lycett SJ, Worobey M, Pybus OG, et al. Origins and evolutionary genomics of the 2009 swine-origin H1N1 influenza A epidemic. Nature. 2009;459:1122–1125. - PubMed
-
- Naffakh N, van der Werf S. April 2009: an outbreak of swine-origin influenza A (H1N1) virus with evidence for human-to-human transmission. Microbes Infect. 2009;11:725–728. - PubMed
-
- Cox RJ, Madhun AS, Hauge S, Sjursen H, Major D, Kuhne M, et al. A phase I clinical trial of a PER.C6 cell grown influenza H7 virus vaccine. Vaccine. 2009;27:1889–1897. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

