Traction forces during collective cell motion
- PMID: 20119479
- PMCID: PMC2799984
- DOI: 10.2976/1.3185785
Traction forces during collective cell motion
Abstract
Collective motion of cell cultures is a process of great interest, as it occurs during morphogenesis, wound healing, and tumor metastasis. During these processes cell cultures move due to the traction forces induced by the individual cells on the surrounding matrix. A recent study [Trepat, et al. (2009). Nat. Phys. 5, 426-430] measured for the first time the traction forces driving collective cell migration and found that they arise throughout the cell culture. The leading 5-10 rows of cell do play a major role in directing the motion of the rest of the culture by having a distinct outwards traction. Fluctuations in the traction forces are an order of magnitude larger than the resultant directional traction at the culture edge and, furthermore, have an exponential distribution. Such exponential distributions are observed for the sizes of adhesion domains within cells, the traction forces produced by single cells, and even in nonbiological nonequilibrium systems, such as sheared granular materials. We discuss these observations and their implications for our understanding of cellular flows within a continuous culture.
Figures
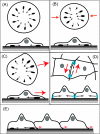
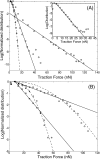
Comment on
- Trepat, et al. (2009). Nat. Phys. 5, 426–430
Similar articles
-
Microfabricated tissues for investigating traction forces involved in cell migration and tissue morphogenesis.Cell Mol Life Sci. 2017 May;74(10):1819-1834. doi: 10.1007/s00018-016-2439-z. Epub 2016 Dec 22. Cell Mol Life Sci. 2017. PMID: 28008471 Free PMC article. Review.
-
Traction Forces Control Cell-Edge Dynamics and Mediate Distance Sensitivity during Cell Polarization.Curr Biol. 2020 May 4;30(9):1762-1769.e5. doi: 10.1016/j.cub.2020.02.078. Epub 2020 Mar 26. Curr Biol. 2020. PMID: 32220324
-
Mechanochemical Coupling and Junctional Forces during Collective Cell Migration.Biophys J. 2019 Jul 9;117(1):170-183. doi: 10.1016/j.bpj.2019.05.020. Epub 2019 May 28. Biophys J. 2019. PMID: 31200935 Free PMC article.
-
Forces to Drive Neuronal Migration Steps.Front Cell Dev Biol. 2020 Sep 1;8:863. doi: 10.3389/fcell.2020.00863. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32984342 Free PMC article. Review.
-
Force generation by groups of migrating bacteria.Proc Natl Acad Sci U S A. 2017 Jul 11;114(28):7266-7271. doi: 10.1073/pnas.1621469114. Epub 2017 Jun 27. Proc Natl Acad Sci U S A. 2017. PMID: 28655845 Free PMC article.
Cited by
-
Onset of nonlinearity in a stochastic model for auto-chemotactic advancing epithelia.Sci Rep. 2016 Sep 27;6:33849. doi: 10.1038/srep33849. Sci Rep. 2016. PMID: 27669998 Free PMC article.
-
Silver Nanoparticles at Low Concentrations Embedded in ECM Promote Endothelial Monolayer Formation and Cell Migration.Int J Mol Sci. 2025 May 16;26(10):4761. doi: 10.3390/ijms26104761. Int J Mol Sci. 2025. PMID: 40429902 Free PMC article.
-
Seeds of Locally Aligned Motion and Stress Coordinate a Collective Cell Migration.Biophys J. 2015 Dec 15;109(12):2492-2500. doi: 10.1016/j.bpj.2015.11.001. Biophys J. 2015. PMID: 26682808 Free PMC article.
-
Classifying collective cancer cell invasion.Nat Cell Biol. 2012 Aug;14(8):777-83. doi: 10.1038/ncb2548. Nat Cell Biol. 2012. PMID: 22854810 Review.
-
Regulation of epithelial cell organization by tuning cell-substrate adhesion.Integr Biol (Camb). 2015 Oct;7(10):1228-41. doi: 10.1039/c5ib00196j. Epub 2015 Sep 24. Integr Biol (Camb). 2015. PMID: 26402903 Free PMC article.
References
-
- Balaban, N Q, Schwarz, U S, Riveline, D, Goichberg, P, Tzur, G, Sabanay, I, Mahalu, D, Safran, S, Bershadsky, A, Addadi, L, and Geiger, B (2001). “Force and focal adhesion assembly: a close relationship studied using elastic micro-patterned substrates.” Nat. Cell Biol. NCBIFN 3, 466–472.10.1038/35074532 - DOI - PubMed
LinkOut - more resources
Full Text Sources
