Experimental and computational aspects of signaling mechanisms of spike-timing-dependent plasticity
- PMID: 20119481
- PMCID: PMC2799986
- DOI: 10.2976/1.3137602
Experimental and computational aspects of signaling mechanisms of spike-timing-dependent plasticity
Abstract
STDP (spike-timing-dependent synaptic plasticity) is thought to be a synaptic learning rule that embeds spike-timing information into a specific pattern of synaptic strengths in neuronal circuits, resulting in a memory. STDP consists of bidirectional long-term changes in synaptic strengths. This process includes long-term potentiation and long-term depression, which are dependent on the timing of presynaptic and postsynaptic spikings. In this review, we focus on computational aspects of signaling mechanisms that induce and maintain STDP as a key step toward the definition of a general synaptic learning rule. In addition, we discuss the temporal and spatial aspects of STDP, and the requirement of a homeostatic mechanism of STDP in vivo.
Figures
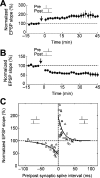

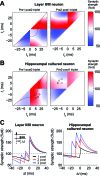
References
LinkOut - more resources
Full Text Sources
