Photochemical tools to study dynamic biological processes
- PMID: 20119482
- PMCID: PMC2799987
- DOI: 10.2976/1.3132954
Photochemical tools to study dynamic biological processes
Abstract
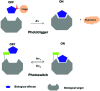
Light-responsive biologically active compounds offer the possibility to study the dynamics of biological processes. Phototriggers and photoswitches have been designed, providing the capability to rapidly cause the initiation of wide range of dynamic biological phenomena. We will discuss, in this article, recent developments in the field of light-triggered chemical tools, specially how two-photon excitation, "caged" fluorophores, and the photoregulation of protein activities in combination with time-resolved x-ray techniques should break new grounds in the understanding of dynamic biological processes.
Figures
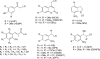


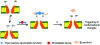
Similar articles
-
From one-photon to two-photon probes: "caged" compounds, actuators, and photoswitches.Angew Chem Int Ed Engl. 2013 Apr 22;52(17):4526-37. doi: 10.1002/anie.201204203. Epub 2013 Feb 18. Angew Chem Int Ed Engl. 2013. PMID: 23417981 Review.
-
Recent developments in reversible photoregulation of oligonucleotide structure and function.Chem Soc Rev. 2017 Feb 20;46(4):1052-1079. doi: 10.1039/c6cs00461j. Chem Soc Rev. 2017. PMID: 28128377 Review.
-
Indigoid Photoswitches: Visible Light Responsive Molecular Tools.Acc Chem Res. 2018 May 15;51(5):1153-1163. doi: 10.1021/acs.accounts.7b00638. Epub 2018 Apr 25. Acc Chem Res. 2018. PMID: 29694014
-
Rationally designed azobenzene photoswitches for efficient two-photon neuronal excitation.Nat Commun. 2019 Feb 22;10(1):907. doi: 10.1038/s41467-019-08796-9. Nat Commun. 2019. PMID: 30796228 Free PMC article.
-
Nanotransducers for Near-Infrared Photoregulation in Biomedicine.Adv Mater. 2019 Aug;31(33):e1901607. doi: 10.1002/adma.201901607. Epub 2019 Jun 14. Adv Mater. 2019. PMID: 31199021 Review.
Cited by
-
Structure-Photoreactivity Studies of BODIPY Photocages: Limitations of the Activation Barrier for Optimizing Photoreactions.J Org Chem. 2024 May 17;89(10):6740-6748. doi: 10.1021/acs.joc.3c02980. Epub 2024 May 2. J Org Chem. 2024. PMID: 38695507 Free PMC article.
-
Rationalizing the environment-dependent photophysical behavior of a DNA luminescent probe by classical and non-adiabatic molecular dynamics simulations.Photochem Photobiol Sci. 2023 Sep;22(9):2081-2092. doi: 10.1007/s43630-023-00431-3. Epub 2023 May 11. Photochem Photobiol Sci. 2023. PMID: 37166569
-
Synthesis and activation of an iron oxide immobilized drug-mimicking reporter under conventional and pulsed X-ray irradiation conditions.RSC Adv. 2020 Jan 21;10(6):3366-3370. doi: 10.1039/c9ra09828c. eCollection 2020 Jan 16. RSC Adv. 2020. PMID: 35497736 Free PMC article.
-
Light-Regulated Angiogenesis via a Phototriggerable VEGF Peptidomimetic.Adv Healthc Mater. 2021 Jul;10(14):e2100488. doi: 10.1002/adhm.202100488. Epub 2021 Jun 10. Adv Healthc Mater. 2021. PMID: 34110713 Free PMC article.
-
Design and Synthesis of a 4-Nitrobromobenzene Derivative Bearing an Ethylene Glycol Tetraacetic Acid Unit for a New Generation of Caged Calcium Compounds with Two-Photon Absorption Properties in the Near-IR Region and Their Application in Vivo.ACS Omega. 2016 Aug 9;1(2):193-201. doi: 10.1021/acsomega.6b00119. eCollection 2016 Aug 31. ACS Omega. 2016. PMID: 31457124 Free PMC article.
References
-
- Adams, S R, Kao, J PY, Grynkiewicz, G, Minta, A, and Tsien, R Y (1988). “Biologically useful chelators that release Ca2+ upon illumination.” J. Am. Chem. Soc. JACSAT 110(10), 3212–3220.10.1021/ja00218a034 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
