Canine distemper virus persistence in demyelinating encephalitis by swift intracellular cell-to-cell spread in astrocytes is controlled by the viral attachment protein
- PMID: 20119836
- PMCID: PMC2849939
- DOI: 10.1007/s00401-010-0644-7
Canine distemper virus persistence in demyelinating encephalitis by swift intracellular cell-to-cell spread in astrocytes is controlled by the viral attachment protein
Abstract
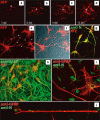
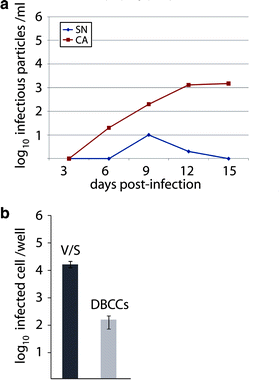
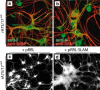
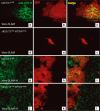
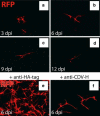
The mechanism of viral persistence, the driving force behind the chronic progression of inflammatory demyelination in canine distemper virus (CDV) infection, is associated with non-cytolytic viral cell-to-cell spread. Here, we studied the molecular mechanisms of viral spread of a recombinant fluorescent protein-expressing virulent CDV in primary canine astrocyte cultures. Time-lapse video microscopy documented that CDV spread was very efficient using cell processes contacting remote target cells. Strikingly, CDV transmission to remote cells could occur in less than 6 h, suggesting that a complete viral cycle with production of extracellular free particles was not essential in enabling CDV to spread in glial cells. Titration experiments and electron microscopy confirmed a very low CDV particle production despite higher titers of membrane-associated viruses. Interestingly, confocal laser microscopy and lentivirus transduction indicated expression and functionality of the viral fusion machinery, consisting of the viral fusion (F) and attachment (H) glycoproteins, at the cell surface. Importantly, using a single-cycle infectious recombinant H-knockout, H-complemented virus, we demonstrated that H, and thus potentially the viral fusion complex, was necessary to enable CDV spread. Furthermore, since we could not detect CD150/SLAM expression in brain cells, the presence of a yet non-identified glial receptor for CDV was suggested. Altogether, our findings indicate that persistence in CDV infection results from intracellular cell-to-cell transmission requiring the CDV-H protein. Viral transfer, happening selectively at the tip of astrocytic processes, may help the virus to cover long distances in the astroglial network, "outrunning" the host's immune response in demyelinating plaques, thus continuously eliciting new lesions.
Figures

Similar articles
-
SLAM- and nectin-4-independent noncytolytic spread of canine distemper virus in astrocytes.J Virol. 2015 May;89(10):5724-33. doi: 10.1128/JVI.00004-15. Epub 2015 Mar 18. J Virol. 2015. PMID: 25787275 Free PMC article.
-
Mechanism of reduction of virus release and cell-cell fusion in persistent canine distemper virus infection.Acta Neuropathol. 2003 Oct;106(4):303-10. doi: 10.1007/s00401-003-0731-0. Epub 2003 Jun 21. Acta Neuropathol. 2003. PMID: 12827396
-
Vimentin-positive astrocytes in canine distemper: a target for canine distemper virus especially in chronic demyelinating lesions?Acta Neuropathol. 2007 Dec;114(6):597-608. doi: 10.1007/s00401-007-0307-5. Epub 2007 Oct 27. Acta Neuropathol. 2007. PMID: 17965866
-
New aspects of the pathogenesis of canine distemper leukoencephalitis.Viruses. 2014 Jul 2;6(7):2571-601. doi: 10.3390/v6072571. Viruses. 2014. PMID: 24992230 Free PMC article. Review.
-
Tropism and molecular pathogenesis of canine distemper virus.Virol J. 2019 Mar 7;16(1):30. doi: 10.1186/s12985-019-1136-6. Virol J. 2019. PMID: 30845967 Free PMC article. Review.
Cited by
-
Measles Virus Fusion Protein: Structure, Function and Inhibition.Viruses. 2016 Apr 21;8(4):112. doi: 10.3390/v8040112. Viruses. 2016. PMID: 27110811 Free PMC article. Review.
-
Canine distemper virus infection leads to an inhibitory phenotype of monocyte-derived dendritic cells in vitro with reduced expression of co-stimulatory molecules and increased interleukin-10 transcription.PLoS One. 2014 Apr 25;9(4):e96121. doi: 10.1371/journal.pone.0096121. eCollection 2014. PLoS One. 2014. PMID: 24769532 Free PMC article.
-
Infectious Progression of Canine Distemper Virus from Circulating Cerebrospinal Fluid into the Central Nervous System.J Virol. 2016 Sep 29;90(20):9285-92. doi: 10.1128/JVI.01337-16. Print 2016 Oct 15. J Virol. 2016. PMID: 27489268 Free PMC article.
-
First report of canine morbillivirus infection of adipose tissue-derived stem cells from dogs with distemper.Vet World. 2022 Jul;15(7):1835-1842. doi: 10.14202/vetworld.2022.1835-1842. Epub 2022 Jul 27. Vet World. 2022. PMID: 36185532 Free PMC article.
-
Canine Distemper Virus in Endangered Species: Species Jump, Clinical Variations, and Vaccination.Pathogens. 2022 Dec 29;12(1):57. doi: 10.3390/pathogens12010057. Pathogens. 2022. PMID: 36678405 Free PMC article. Review.
References
-
- Brettschneider J, Tumani H, Kiechle U, Muche R, Richards G, Lehmensiek V, Ludolph AC, Otto M. IgG antibodies against measles, rubella, and varicella zoster virus predict conversion to multiple sclerosis in clinically isolated syndrome. PLoS One. 2009;4:e7638. doi: 10.1371/journal.pone.0007638. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

