Reversible detection of heparin and other polyanions by pulsed chronopotentiometric polymer membrane electrode
- PMID: 20121058
- PMCID: PMC2992876
- DOI: 10.1021/ac902836e
Reversible detection of heparin and other polyanions by pulsed chronopotentiometric polymer membrane electrode
Abstract
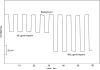
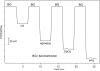
The first fully reversible polymeric membrane-based sensor for the anticoagulant heparin and other polyanions using a pulsed chronopotentiometry (pulstrode) measurement mode is reported. Polymeric membranes containing a lipophilic inert salt of the form R(+)R(-) (where R(+) and R(-) are tridodecylmethylammonium (TDMA(+)) and dinonylnaphthalene sulfonate (DNNS(-)), respectively) are used to suppress unwanted spontaneous ion extractions under zero-current equilibrium conditions. An anodic galvanostatic current pulse applied across the membrane perturbs the equilibrium lipophilic ion distribution within the membrane phase in such a way that anions/polyanions are extracted into the membrane from the sample. The membrane is then subjected to an open-circuit zero current state for a short period, and finally a 0 V vs reference electrode potentiostatic pulse is applied to restore the membrane to its initial full equilibrium condition. Potentials are sampled as average values during the last 10% of the 0.5 s open circuit phase of the measurement cycle. Fully reversible and reproducible electromotive force (emf) responses are observed for heparin, pentosan polysulfate (PPS), chondroitin sulfate (CS), and oversulfated chondroitin sulfate (OSCS), with the magnitude of the potentiometric response proportional to charge density of the polyanions. The sensor provides an emf response related to heparin concentrations in the range of 1-20 U/mL. The responses to variations in heparin levels and toward other polyanions of the pulstrode configuration are analogous to the already established single-use, nonreversible potentiometric polyion sensors based on membranes doped only with the lipophilic anion exchanger TDMA(+).
Figures

Similar articles
-
Polyion selective polymeric membrane-based pulstrode as a detector in flow-injection analysis.Anal Chem. 2014 Apr 15;86(8):4041-6. doi: 10.1021/ac500567g. Epub 2014 Apr 1. Anal Chem. 2014. PMID: 24650129 Free PMC article.
-
Reversible Electrochemical Sensor for Detection of High-Charge Density Polyanion Contaminants in Heparin.Anal Chem. 2015 Nov 17;87(22):11537-43. doi: 10.1021/acs.analchem.5b03347. Epub 2015 Oct 28. Anal Chem. 2015. PMID: 26485655
-
Detection of high-charge density polyanion contaminants in biomedical heparin preparations using potentiometric polyanion sensors.Anal Chem. 2008 Dec 15;80(24):9845-7. doi: 10.1021/ac801879t. Anal Chem. 2008. PMID: 19007240 Free PMC article.
-
Electrochemical heparin sensing at liquid/liquid interfaces and polymeric membranes.Anal Bioanal Chem. 2011 Jan;399(2):571-9. doi: 10.1007/s00216-010-4056-2. Epub 2010 Aug 5. Anal Bioanal Chem. 2011. PMID: 20686753 Review.
-
Polymer membrane-based ion-, gas- and bio-selective potentiometric sensors.Biosens Bioelectron. 1993;8(1):1-38. doi: 10.1016/0956-5663(93)80041-m. Biosens Bioelectron. 1993. PMID: 8499085 Review.
Cited by
-
Detecting Levels of Polyquaternium-10 (PQ-10) via Potentiometric Titration with Dextran Sulphate and Monitoring the Equivalence Point with a Polymeric Membrane-Based Polyion Sensor.Anal Methods. 2016 Aug 7;8(29):5806-5811. doi: 10.1039/C6AY01748G. Epub 2016 Jul 4. Anal Methods. 2016. PMID: 28018490 Free PMC article.
-
Detection of protease activities by flash chronopotentiometry using a reversible polycation-sensitive polymeric membrane electrode.Anal Biochem. 2011 Sep 1;416(1):67-73. doi: 10.1016/j.ab.2011.04.036. Epub 2011 Apr 29. Anal Biochem. 2011. PMID: 21601559 Free PMC article.
-
A "turn on" fluorescent probe for heparin and its oversulfated chondroitin sulfate contaminant.Chem Sci. 2015 Nov 1;6(11):6361-6366. doi: 10.1039/c5sc01675d. Epub 2015 Jul 23. Chem Sci. 2015. PMID: 30090254 Free PMC article.
-
Polyion selective polymeric membrane-based pulstrode as a detector in flow-injection analysis.Anal Chem. 2014 Apr 15;86(8):4041-6. doi: 10.1021/ac500567g. Epub 2014 Apr 1. Anal Chem. 2014. PMID: 24650129 Free PMC article.
-
Glucose-sensitive polyelectrolyte nanocapsules based on layer-by-layer technique for protein drug delivery.J Mater Sci Mater Med. 2014 Jan;25(1):121-9. doi: 10.1007/s10856-013-5055-6. Epub 2013 Sep 26. J Mater Sci Mater Med. 2014. PMID: 24068543
References
-
- Guerrini M, Beccati D, Shriver Z, Naggi A, Viswanathan K, Bisio A, Capila I, Lansing JC, Guglieri S, Fraser B, Al-Hakim A, Gunay NS, Zhang Z, Robinson L, Buhse L, Nasr M, Woodcock J, Langer R, Venkataraman G, Linhardt RJ, Casu B, Torri G, Sasisekharan R. Nat. Biotechnol. 2008;26:669–675. - PMC - PubMed
-
- Ma SC, Yang VC, Meyerhoff ME. Anal. Chem. 1992;64:694–697. - PubMed
-
- Ma SC, Yang VC, Fu B, Meyerhoff ME. Anal. Chem. 1993;65:2078–2084. - PubMed
-
- Fu B, Bakker E, Yun JH, Yang VC, Meyerhoff ME. Anal. Chem. 1994;66:2250–2259. - PubMed
-
- Meyerhoff ME, Yang VC, Wahr JA, Lee LM, Yun JH, Fu B, Bakker E. Clin. Chem. 1995;41:1355–1356.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

