Evolving carbapenemases: can medicinal chemists advance one step ahead of the coming storm?
- PMID: 20121112
- PMCID: PMC2855403
- DOI: 10.1021/jm9012938
Evolving carbapenemases: can medicinal chemists advance one step ahead of the coming storm?
Figures
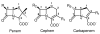
Similar articles
-
Crystal structures of KPC-2 β-lactamase in complex with 3-nitrophenyl boronic acid and the penam sulfone PSR-3-226.Antimicrob Agents Chemother. 2012 May;56(5):2713-8. doi: 10.1128/AAC.06099-11. Epub 2012 Feb 13. Antimicrob Agents Chemother. 2012. PMID: 22330909 Free PMC article.
-
Local interactions with the Glu166 base and the conformation of an active site loop play key roles in carbapenem hydrolysis by the KPC-2 β-lactamase.J Biol Chem. 2021 Jan-Jun;296:100799. doi: 10.1016/j.jbc.2021.100799. Epub 2021 May 20. J Biol Chem. 2021. PMID: 34022225 Free PMC article.
-
Klebsiella pneumoniae Carbapenemase-2 (KPC-2), Substitutions at Ambler Position Asp179, and Resistance to Ceftazidime-Avibactam: Unique Antibiotic-Resistant Phenotypes Emerge from β-Lactamase Protein Engineering.mBio. 2017 Oct 31;8(5):e00528-17. doi: 10.1128/mBio.00528-17. mBio. 2017. PMID: 29089425 Free PMC article.
-
Ten Years with New Delhi Metallo-β-lactamase-1 (NDM-1): From Structural Insights to Inhibitor Design.ACS Infect Dis. 2019 Jan 11;5(1):9-34. doi: 10.1021/acsinfecdis.8b00247. Epub 2018 Nov 28. ACS Infect Dis. 2019. PMID: 30421910 Review.
-
Decoding the Structural Basis For Carbapenem Hydrolysis By Class A β-lactamases: Fishing For A Pharmacophore.Curr Drug Targets. 2016;17(9):983-1005. doi: 10.2174/1389450116666151001104448. Curr Drug Targets. 2016. PMID: 26424401 Review.
Cited by
-
Protein variants form a system of networks: microdiversity of IMP metallo-beta-lactamases.PLoS One. 2014 Jul 11;9(7):e101813. doi: 10.1371/journal.pone.0101813. eCollection 2014. PLoS One. 2014. PMID: 25013948 Free PMC article.
-
Carbamylmethyl Mercaptoacetate Thioether: A Novel Scaffold for the Development of L1 Metallo-β-lactamase Inhibitors.ACS Med Chem Lett. 2017 Apr 24;8(5):527-532. doi: 10.1021/acsmedchemlett.7b00058. eCollection 2017 May 11. ACS Med Chem Lett. 2017. PMID: 28523105 Free PMC article.
-
On drug discovery against infectious diseases and academic medicinal chemistry contributions.Beilstein J Org Chem. 2022 Sep 29;18:1355-1378. doi: 10.3762/bjoc.18.141. eCollection 2022. Beilstein J Org Chem. 2022. PMID: 36247982 Free PMC article.
-
Elucidating the Role of Residue 67 in IMP-Type Metallo-β-Lactamase Evolution.Antimicrob Agents Chemother. 2015 Dec;59(12):7299-307. doi: 10.1128/AAC.01651-15. Epub 2015 Sep 14. Antimicrob Agents Chemother. 2015. PMID: 26369960 Free PMC article.
-
Assay platform for clinically relevant metallo-β-lactamases.J Med Chem. 2013 Sep 12;56(17):6945-53. doi: 10.1021/jm400769b. Epub 2013 Aug 16. J Med Chem. 2013. PMID: 23898798 Free PMC article.
References
-
- Rossolini GM, Mantengoli E. Treatment and control of severe infections caused by multiresistant Pseudomonas aeruginosan. Clin. Microbiol. Infect. 2005;11(Suppl 4):17–32. - PubMed
-
- Karageorgopoulos DE, Falagas ME. Current control and treatment of multidrug-resistant Acinetobacter baumannii infections. Lancet Infect. Dis. 2008;8:751–762. - PubMed
-
- Ramphal R, Ambrose PG. Extended-spectrum β-lactamases and clinical outcomes: current data. Clin. Infect. Dis. 2006;42(Suppl 4):S164–172. - PubMed
-
- Isturiz R. Global resistance trends and the potential impact on empirical therapy. Int. J. Antimicrob. Agents. 2008;32(Suppl 4):S201–206. - PubMed
-
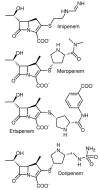
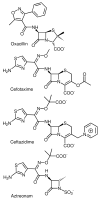
- Barza M. Imipenem: first of a new class of β-lactam antibiotics. Ann. Intern. Med. 1985;103:552–560. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

