Near-stoichiometric conversion of H(2)O(2) to Fe(IV)=O at a nonheme iron(II) center. Insights into the O-O bond cleavage step
- PMID: 20121136
- PMCID: PMC2823852
- DOI: 10.1021/ja9101908
Near-stoichiometric conversion of H(2)O(2) to Fe(IV)=O at a nonheme iron(II) center. Insights into the O-O bond cleavage step
Abstract
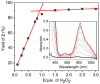
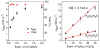
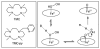
Near-quantitative formation of an oxoiron(IV) intermediate [Fe(IV)(O)(TMC)(CH(3)CN)](2+) (2) from stoichiometric H(2)O(2) was achieved with [Fe(II)(TMC)](2+) (1) (TMC = 1,4,8,11-tetramethyl-1,4,8,11-tetraaza-cyclotetradecane). This important outcome is best rationalized by invoking a direct reaction between 1 and H(2)O(2) followed by a heterolytic O-O bond cleavage facilitated by an acid-base catalyst (2,6-lutidine in our case). A sizable H/D KIE of 3.7 was observed for the formation of 2, emphasizing the importance of proton transfer in the cleavage step. Pyridines with different pK(a) values were also investigated, and less basic pyridines were found to function less effectively than 2,6-lutidine. This study demonstrates that the reaction of Fe(II) with H(2)O(2) to form Fe(IV)= O can be quite facile. Two factors promote the near-stoichiometric conversion of H(2)O(2) to Fe(IV)=O in this case: (a) the low reactivity between 1 and 2 and (b) the poor H-atom abstracting ability of 2, which inhibits subsequent reaction with residual H(2)O(2). Both factors inhibit formation of the Fe(III) byproduct commonly found in reactions of Fe(II) complexes with H(2)O(2). These results may shed light into the nature of the O-O bond cleaving step in the activation of dioxygen by nonheme iron enzymes and in the first step of the Fenton reaction.
Figures
References
-
- Costas M, Mehn MP, Jensen MP, Que L., Jr Chem Rev. 2004;104:939–986. - PubMed
- Eser BE, Barr EW, Frantom PA, Saleh L, Bollinger JM, Jr, Krebs C, Fitzpatrick PF. J Am Chem Soc. 2007;129:11334–11335. - PMC - PubMed
- Chow MS, Eser BE, Wilson SA, Hodgson KO, Hedman B, Fitzpatrick PF, Solomon EI. J Am Chem Soc. 2009;131:7685–7698. - PMC - PubMed
- Schenk WA. Angew Chem Int Ed. 2000;39:3409–3411. - PubMed
- Brown-Marshall CD, Diebold AR, Solomon EI. Biochemistry. 2010 doi: 10.1021/bi901772w. Online Early Access. - DOI - PMC - PubMed
-
-
TMC = 1,4,8,11-tetramethyl-1,4,8,11-tetraazacyclotetradecane; TMC-Py = 1-(2-pyridylmethyl)-4,8,11-trimethyl-1,4,8,11-tetraazacyclotetradecane.
-
-
- Rohde JU, In JH, Lim MH, Brennessel WW, Bukowski MR, Stubna A, Münck E, Nam W, Que L., Jr Science. 2003;299:1037–1039. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

