Iodine/palladium approaches to the synthesis of polyheterocyclic compounds
- PMID: 20121269
- PMCID: PMC2832103
- DOI: 10.1021/jo902639f
Iodine/palladium approaches to the synthesis of polyheterocyclic compounds
Abstract
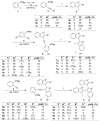
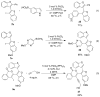
A simple, straightforward strategy for the synthesis of polyheterocyclic compounds (PHCs) is reported, which involves iterative cycles of palladium-catalyzed Sonogashira coupling, followed by iodocyclization using I(2) or ICl. A variety of heterocyclic units, including benzofurans, benzothiophenes, indoles, and isocoumarins, can be efficiently incorporated under mild reaction conditions. In addition, variations of this strategy afford a variety of linked and fused PHCs.
Figures
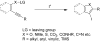

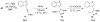
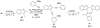


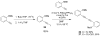
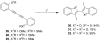
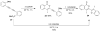

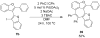
Similar articles
-
Iodocyclization, followed by palladium-catalyzed coupling: a versatile strategy for heterocyclic library construction.Comb Chem High Throughput Screen. 2012 Jul;15(6):451-72. doi: 10.2174/138620712800563927. Comb Chem High Throughput Screen. 2012. PMID: 22272662 Review.
-
Synthesis of 2,5-disubstituted 3-iodofurans via palladium-catalyzed coupling and iodocyclization of terminal alkynes.J Org Chem. 2011 Feb 18;76(4):1134-9. doi: 10.1021/jo1023987. Epub 2011 Jan 14. J Org Chem. 2011. PMID: 21235260
-
Palladium catalyzed N-H bond insertion and intramolecular cyclization cascade: the divergent synthesis of heterocyclics.Org Biomol Chem. 2014 Apr 28;12(16):2533-7. doi: 10.1039/c4ob00001c. Org Biomol Chem. 2014. PMID: 24633071
-
Synthesis of 3-aryl-8-oxo-5,6,7,8-tetrahydroindolizines via a palladium-catalyzed arylation and heteroarylation.J Org Chem. 2009 Apr 17;74(8):3160-3. doi: 10.1021/jo802768n. J Org Chem. 2009. PMID: 19296664
-
Synthesis of heterocycles via palladium-catalyzed oxidative addition.Chem Rev. 2006 Nov;106(11):4644-80. doi: 10.1021/cr0683966. Chem Rev. 2006. PMID: 17091931 Review. No abstract available.
Cited by
-
Nucleophilic C-H Etherification of Heteroarenes Enabled by Base-Catalyzed Halogen Transfer.J Am Chem Soc. 2021 Aug 18;143(32):12480-12486. doi: 10.1021/jacs.1c06481. Epub 2021 Aug 4. J Am Chem Soc. 2021. PMID: 34347457 Free PMC article.
-
Discovery of desketoraloxifene analogues as inhibitors of mammalian, Pseudomonas aeruginosa, and NAPE phospholipase D enzymes.ACS Chem Biol. 2015 Feb 20;10(2):421-32. doi: 10.1021/cb500828m. Epub 2014 Nov 19. ACS Chem Biol. 2015. PMID: 25384256 Free PMC article.
-
Choline Chloride/Urea - Promoted Iodocyclization of 3-Alkynylthiophene-2-carboxamides: A Green Synthesis of 4-Iodo-7H-thieno[2,3-c]pyran-7-imines and Their Coupling Reactions in Deep Eutectic Solvents.Chemistry. 2025 May 14;31(27):e202500081. doi: 10.1002/chem.202500081. Epub 2025 Apr 16. Chemistry. 2025. PMID: 40156888 Free PMC article.
-
Regio- and stereoselective synthesis of cyclic imidates via electrophilic cyclization of 2-(1-alkynyl)benzamides. A correction.J Org Chem. 2012 Dec 7;77(23):10938-44. doi: 10.1021/jo301958q. Epub 2012 Nov 15. J Org Chem. 2012. PMID: 23110553 Free PMC article.
-
2-Amino-4-methyl-5-phenylethyl substituted-7-N-benzyl-pyrrolo[2,3-d]pyrimidines as novel antitumor antimitotic agents that also reverse tumor resistance.Bioorg Med Chem. 2011 Jul 15;19(14):4355-65. doi: 10.1016/j.bmc.2011.05.030. Epub 2011 May 23. Bioorg Med Chem. 2011. PMID: 21680190 Free PMC article.
References
-
- Arcadi A, Cacchi S, Fabrizi G, Marinelli F, Moro L. Synlett. 1999:1432.
- Yue D, Yao T, Larock RC. J Org Chem. 2005;70:10292. - PMC - PubMed
- Yue D, Yao T, Larock RC. J Comb Chem. 2005;7:809. - PubMed
- Manarin F, Roehrs JA, Gay RM, Brandão R, Menezes PH, Nogueira CW, Zeni G. J Org Chem. 2009;74:2153. - PubMed
-
- Sniady A, Wheeler KA, Dembinski R. Org Lett. 2005;7:1769. - PubMed
- Yao T, Zhang X, Larock RC. J Org Chem. 2005;70:7679. - PubMed
- Liu Y, Zhou S. Org Lett. 2005;7:4609. - PubMed
- Yao T, Zhang X, Larock RC. J Am Chem Soc. 2004;126:11164. - PubMed
- Bew SP, El-Taeb GMM, Jones S, Knight DW, Tan W. Eur J Org Chem. 2007:5759.
- Arimitsu S, Jacobsen JM, Hammond GB. J Org Chem. 2008;73:2886. - PubMed
- Huang X, Fu W, Miao M. Tetrahedron Lett. 2008;49:2359.
- Okitsu T, Nakazawa D, Taniguchi R, Wada A. Org Lett. 2008;10:4967. - PubMed
-
- Flynn BL, Flynn GP, Hamel E, Jung MK. Bioorg Med Chem Lett. 2001;11:2341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

