Ultrafast dynamics of diatomic ligand binding to nitrophorin 4
- PMID: 20121274
- PMCID: PMC2835772
- DOI: 10.1021/ja910005b
Ultrafast dynamics of diatomic ligand binding to nitrophorin 4
Abstract
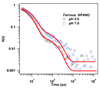
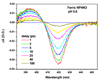
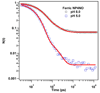
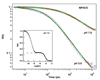
Nitrophorin 4 (NP4) is a heme protein that stores and delivers nitric oxide (NO) through pH-sensitive conformational change. This protein uses the ferric state of a highly ruffled heme to bind NO tightly at low pH and release it at high pH. In this work, the rebinding kinetics of NO and CO to NP4 are investigated as a function of iron oxidation state and the acidity of the environment. The geminate recombination process of NO to ferrous NP4 at both pH 5 and pH 7 is dominated by a single approximately 7 ps kinetic phase that we attribute to the rebinding of NO directly from the distal pocket. The lack of pH dependence explains in part why NP4 cannot use the ferrous state to fulfill its function. The kinetic response of ferric NP4NO shows two distinct phases. The relative geminate amplitude of the slower phase increases dramatically as the pH is raised from 5 to 8. We assign the fast phase of NO rebinding to a conformation of the ferric protein with a closed hydrophobic pocket. The slow phase is assigned to the protein in an open conformation with a more hydrophilic heme pocket environment. Analysis of the ultrafast kinetics finds the equilibrium off-rate of NO to be proportional to the open state population as well as the pH-dependent amplitude of escape from the open pocket. When both factors are considered, the off-rate increases by more than an order of magnitude as the pH changes from 5 to 8. The recombination of CO to ferrous NP4 is observed to have a large nonexponential geminate amplitude with rebinding time scales of approximately 10(-11)-10(-9) s at pH 5 and approximately 10(-10)-10(-8) s at pH 7. The nonexponential CO rebinding kinetics at both pH 5 and pH 7 are accounted for using a simple model that has proven effective for understanding CO binding in a variety of other heme systems (Ye, X.; et al. Proc. Natl. Acad. Sci. U.S.A. 2007, 104, 14682).
Figures


Similar articles
-
Structural dynamics controls nitric oxide affinity in nitrophorin 4.J Biol Chem. 2004 Sep 17;279(38):39401-7. doi: 10.1074/jbc.M406178200. Epub 2004 Jul 16. J Biol Chem. 2004. PMID: 15258143
-
Heterogeneous kinetics of the carbon monoxide association and dissociation reaction to nitrophorin 4 and 7 coincide with structural heterogeneity of the gate-loop.J Am Chem Soc. 2012 Jun 20;134(24):9986-98. doi: 10.1021/ja2121662. Epub 2012 Jun 6. J Am Chem Soc. 2012. PMID: 22594621
-
Kinetics and computational studies of ligand migration in nitrophorin 7 and its Δ1-3 mutant.Biochim Biophys Acta. 2013 Sep;1834(9):1711-21. doi: 10.1016/j.bbapap.2013.04.009. Epub 2013 Apr 26. Biochim Biophys Acta. 2013. PMID: 23624263
-
Nitrophorins: nitrite disproportionation reaction and other novel functionalities of insect heme-based nitric oxide transport proteins.IUBMB Life. 2011 May;63(5):304-12. doi: 10.1002/iub.451. Epub 2011 Apr 13. IUBMB Life. 2011. PMID: 21491557 Review.
-
Ligand dynamics in heme proteins observed by Fourier transform infrared spectroscopy at cryogenic temperatures.Methods Enzymol. 2008;437:347-78. doi: 10.1016/S0076-6879(07)37018-3. Methods Enzymol. 2008. PMID: 18433637 Review.
Cited by
-
Motion of proximal histidine and structural allosteric transition in soluble guanylate cyclase.Proc Natl Acad Sci U S A. 2015 Apr 7;112(14):E1697-704. doi: 10.1073/pnas.1423098112. Epub 2015 Mar 23. Proc Natl Acad Sci U S A. 2015. PMID: 25831539 Free PMC article.
-
Effect of DNA binding on geminate CO recombination kinetics in CO-sensing transcription factor CooA.J Biol Chem. 2012 Jun 22;287(26):21729-40. doi: 10.1074/jbc.M112.345090. Epub 2012 Apr 28. J Biol Chem. 2012. PMID: 22544803 Free PMC article.
-
Ultrafast CO Kinetics in Heme Proteins: Adiabatic Ligand Binding and Heavy Atom Tunneling.J Am Chem Soc. 2017 Nov 8;139(44):15738-15747. doi: 10.1021/jacs.7b07507. Epub 2017 Oct 24. J Am Chem Soc. 2017. PMID: 28984134 Free PMC article.
-
Electrostatic Tuning of the Ligand Binding Mechanism by Glu27 in Nitrophorin 7.Sci Rep. 2018 Jul 18;8(1):10855. doi: 10.1038/s41598-018-29182-3. Sci Rep. 2018. PMID: 30022039 Free PMC article.
-
Following ligand migration pathways from picoseconds to milliseconds in type II truncated hemoglobin from Thermobifida fusca.PLoS One. 2012;7(7):e39884. doi: 10.1371/journal.pone.0039884. Epub 2012 Jul 6. PLoS One. 2012. PMID: 22792194 Free PMC article.
References
-
- Antonini E, Brunori M. Hemoglobin and Myoglobin in their Reactions with Ligands. Amsterdam-London, The Netherlands: North-Holland Publishing Co; 1971.
-
- Ribeiro JM, Hazzard JM, Nussenzveig RH, Champagne DE, Walker FA. Science. 1993;260:539–541. - PubMed
-
- Ortiz de Montellano PR. CYTOCHROME P450 : Structure, Mechanism and Biochemistry. 3rd Edition. New York: Kluwer Academic/Plenum Publishers; 2005.
-
- Li H, Poulos TL. J. Inorg. Biochem. 2005;99:293–305. - PubMed
-
- Rousseau DL, Li D, Couture M, Yeh SR. J. Inorg. Biochem. 2005;99:306–323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

