Hydrogen bond migration between molecular sites observed with ultrafast 2D IR chemical exchange spectroscopy
- PMID: 20121275
- PMCID: PMC2838732
- DOI: 10.1021/jp911452z
Hydrogen bond migration between molecular sites observed with ultrafast 2D IR chemical exchange spectroscopy
Abstract
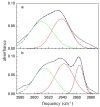
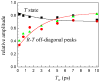
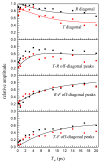
Hydrogen-bonded complexes between phenol and phenylacetylene are studied using ultrafast two-dimensional infrared (2D IR) chemical exchange spectroscopy. Phenylacetylene has two possible pi hydrogen bonding acceptor sites (phenyl or acetylene) that compete for hydrogen bond donors in solution at room temperature. The OD stretch frequency of deuterated phenol is sensitive to which acceptor site it is bound. The appearance of off-diagonal peaks between the two vibrational frequencies in the 2D IR spectrum reports on the exchange process between the two competitive hydrogen-bonding sites of phenol-phenylacetylene complexes in the neat phenylacetylene solvent. The chemical exchange process occurs in approximately 5 ps and is assigned to direct hydrogen bond migration along the phenylacetylene molecule. Other nonmigration mechanisms are ruled out by performing 2D IR experiments on phenol dissolved in the phenylacetylene/carbon tetrachloride mixed solvent. The observation of direct hydrogen bond migration can have implications for macromolecular systems.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

