A model of redox kinetics implicates the thiol proteome in cellular hydrogen peroxide responses
- PMID: 20121341
- PMCID: PMC2935341
- DOI: 10.1089/ars.2009.2968
A model of redox kinetics implicates the thiol proteome in cellular hydrogen peroxide responses
Abstract
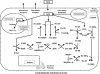
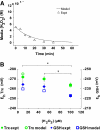
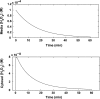
Hydrogen peroxide is appreciated as a cellular signaling molecule with second-messenger properties, yet the mechanisms by which the cell protects against intracellular H(2)O(2) accumulation are not fully understood. We introduce a network model of H(2)O(2) clearance that includes the pseudo-enzymatic oxidative turnover of protein thiols, the enzymatic actions of catalase, glutathione peroxidase, peroxiredoxin, and glutaredoxin, and the redox reactions of thioredoxin and glutathione. Simulations reproduced experimental observations of the rapid and transient oxidation of glutathione and the rapid, sustained oxidation of thioredoxin on exposure to extracellular H(2)O(2). The model correctly predicted early oxidation profiles for the glutathione and thioredoxin redox couples across a range of initial extracellular [H(2)O(2)] and highlights the importance of cytoplasmic membrane permeability to the cellular defense against exogenous sources of H(2)O(2). The protein oxidation profile predicted by the model suggests that approximately 10% of intracellular protein thiols react with hydrogen peroxide at substantial rates, with a majority of these proteins forming protein disulfides as opposed to protein S-glutathionylated adducts. A steady-state flux analysis predicted an unequal distribution of the intracellular anti-oxidative burden between thioredoxin-dependent and glutathione-dependent antioxidant pathways, with the former contributing the majority of the cellular antioxidant defense due to peroxiredoxins and protein disulfides.
Figures


References
-
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. - PubMed
-
- Akerboom TP. Bilzer M. Sies H. The relationship of biliary glutathione disulfide efflux and intracellular glutathione disulfide content in perfused rat liver. J Biol Chem. 1982;257:4248–4252. - PubMed
-
- Antunes F. Cadenas E. Estimation of H2O2 gradients across biomembranes. FEBS Lett. 2000;475:121–126. - PubMed
-
- Arner ES. Zhong L. Holmgren A. Preparation and assay of mammalian thioredoxin and thioredoxin reductase. Methods Enzymol. 1999;300:226–239. - PubMed
-
- Bienert GP. Schjoerring JK. Jahn TP. Membrane transport of hydrogen peroxide. Biochim Biophys Acta. 2006;1758:994–1003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

