Substitution of nevirapine because of efavirenz toxicity in AIDS clinical trials group A5095
- PMID: 20121419
- PMCID: PMC2975665
- DOI: 10.1086/650539
Substitution of nevirapine because of efavirenz toxicity in AIDS clinical trials group A5095
Abstract
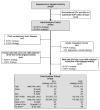
In AIDS Clinical Trials Group A5095, 9% of participants who experienced an adverse event related to efavirenz substituted nevirapine. Most adverse events resolved; 15 participants ultimately discontinued nevirapine therapy. Grade 3/4 hepatotoxicity was observed in 14% of individuals who substituted nevirapine, compared with 6% who continued efavirenz therapy. Substitution of nevirapine because of efavirenz toxicity was generally safe and efficacious. Clinical trials registration. NCT00013520 .
Conflict of interest statement
Figures
Comment in
-
Switching from efavirenz to nevirapine.Clin Infect Dis. 2010 Aug 1;51(3):365; author reply 365-6. doi: 10.1086/654803. Clin Infect Dis. 2010. PMID: 20578877 No abstract available.
Similar articles
-
Long-term effectiveness of first-line non-nucleoside reverse transcriptase inhibitor (NNRTI)-based antiretroviral therapy in Ghana.J Antimicrob Chemother. 2014 Jan;69(1):254-61. doi: 10.1093/jac/dkt336. Epub 2013 Sep 3. J Antimicrob Chemother. 2014. PMID: 24003181
-
Efficacy and safety of dihydroartemisinin-piperaquine for treatment of Plasmodium falciparum uncomplicated malaria in adult patients on antiretroviral therapy in Malawi and Mozambique: an open label non-randomized interventional trial.Malar J. 2019 Aug 20;18(1):277. doi: 10.1186/s12936-019-2909-5. Malar J. 2019. PMID: 31429785 Free PMC article. Clinical Trial.
-
Pharmacokinetics of rifampin and isoniazid in tuberculosis-HIV-coinfected patients receiving nevirapine- or efavirenz-based antiretroviral treatment.Antimicrob Agents Chemother. 2014 Jun;58(6):3182-90. doi: 10.1128/AAC.02379-13. Epub 2014 Mar 24. Antimicrob Agents Chemother. 2014. PMID: 24663014 Free PMC article. Clinical Trial.
-
Is it safe to switch between efavirenz and nevirapine in the event of toxicity?Lancet Infect Dis. 2007 Nov;7(11):733-8. doi: 10.1016/S1473-3099(07)70262-1. Lancet Infect Dis. 2007. PMID: 17961859 Review.
-
Pharmacogenetics of CYP2B6, CYP2A6 and UGT2B7 in HIV treatment in African populations: focus on efavirenz and nevirapine.Drug Metab Rev. 2015 May;47(2):111-23. doi: 10.3109/03602532.2014.982864. Epub 2014 Nov 13. Drug Metab Rev. 2015. PMID: 25391641 Review.
Cited by
-
Baseline predictors of efavirenz-containing antiretroviral regimen adverse experiences.Pharmacoepidemiol Drug Saf. 2014 Jul;23(7):773-7. doi: 10.1002/pds.3615. Epub 2014 Mar 24. Pharmacoepidemiol Drug Saf. 2014. PMID: 24664872 Free PMC article.
-
Perspectives of healthcare professionals of the neuropsychiatric side effects associated with efavirenz and its management.Health SA. 2018 Oct 9;23:1076. doi: 10.4102/hsag.v23i0.1076. eCollection 2018. Health SA. 2018. PMID: 31934375 Free PMC article.
-
Generic and low dose antiretroviral therapy in adults and children: implication for scaling up treatment in resource limited settings.AIDS Res Ther. 2010 Jun 23;7:18. doi: 10.1186/1742-6405-7-18. AIDS Res Ther. 2010. PMID: 20569473 Free PMC article.
-
Influence of efavirenz pharmacokinetics and pharmacogenetics on neuropsychological disorders in Ugandan HIV-positive patients with or without tuberculosis: a prospective cohort study.BMC Infect Dis. 2013 Jun 4;13:261. doi: 10.1186/1471-2334-13-261. BMC Infect Dis. 2013. PMID: 23734829 Free PMC article.
-
Efavirenz-induced gynecomastia in a prepubertal girl with human immunodeficiency virus infection: a case report.BMC Pediatr. 2013 Aug 13;13:120. doi: 10.1186/1471-2431-13-120. BMC Pediatr. 2013. PMID: 23941256 Free PMC article.
References
-
- Mehta U, Maartens G. Is it safe to switch between efavirenz and nevirapine in the event of toxicity? Lancet Infect Dis. 2007;7:733–738. - PubMed
-
- Ward DJ, Curtin JM. Switch from efavirenz to nevirapine associated with resolution of efavirenz-related neuropsychiatric adverse events and improvement in lipid profiles. AIDS Patient Care STDS. 2006;20(8):542–548. - PubMed
-
- FDA public health advisory for nevirapine (Viramune) [Accessed 12 October 2009]. Last updated 30 April 2009. http://www.fda.gov/Drugs/DrugSafety/PublicHealthAdvisories/ucm051674.htm.
-
- Ena J, Leach A, Nguyen P. Switching from suppressive protease inhibitor-based regimens to nevirapine-based regimens: a meta-analysis of randomized controlled trials. HIV Medicine. 2008;9:747–756. - PubMed
-
- Kesselring AM, Wit FW, Sabin CA, et al. Risk factors for treatment-limiting toxicities in patients starting nevirapine-containing antiretroviral therapy. AIDS. 2009;23(13):1689–1699. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UL1 RR024996/RR/NCRR NIH HHS/United States
- AI38858/AI/NIAID NIH HHS/United States
- K24 AI051966/AI/NIAID NIH HHS/United States
- AI068636/AI/NIAID NIH HHS/United States
- UL1-RR024996/RR/NCRR NIH HHS/United States
- AI27659/AI/NIAID NIH HHS/United States
- UM1 AI069419/AI/NIAID NIH HHS/United States
- AI 51966/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- U01 AI038858/AI/NIAID NIH HHS/United States
- U01 AI068636/AI/NIAID NIH HHS/United States
- AI 69419/AI/NIAID NIH HHS/United States
- U01 AI027659/AI/NIAID NIH HHS/United States
- U01 AI069419/AI/NIAID NIH HHS/United States

