Rat parotid gland cell differentiation in three-dimensional culture
- PMID: 20121592
- PMCID: PMC2943407
- DOI: 10.1089/ten.TEC.2009.0438
Rat parotid gland cell differentiation in three-dimensional culture
Abstract
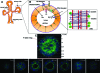
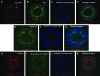
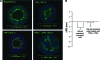
The use of polarized salivary gland cell monolayers has contributed to our understanding of salivary gland physiology. However, these cell models are not representative of glandular epithelium in vivo, and, therefore, are not ideal for investigating salivary epithelial functions. The current study has developed a three-dimensional (3D) cell culture model for rat Par-C10 parotid gland cells that forms differentiated acinar-like spheres on Matrigel. These 3D Par-C10 acinar-like spheres display characteristics similar to differentiated acini in salivary glands, including cell polarization, tight junction (TJ) formation required to maintain transepithelial potential difference, basolateral expression of aquaporin-3 and Na+/K+/2Cl- cotransporter-1, and responsiveness to the muscarinic receptor agonist carbachol that is decreased by the anion channel blocker diphenylamine-2-carboxylic acid or chloride replacement with gluconate. Incubation of the spheres in the hypertonic medium increased the expression level of the water channel aquaporin-5. Further, the proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma induced alterations in TJ integrity in the acinar-like spheres without affecting individual cell viability, suggesting that cytokines may affect salivary gland function by altering TJ integrity. Thus, 3D Par-C10 acinar-like spheres represent a novel in vitro model to study physiological and pathophysiological functions of differentiated acini.
Figures




Similar articles
-
Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma alter tight junction structure and function in the rat parotid gland Par-C10 cell line.Am J Physiol Cell Physiol. 2008 Nov;295(5):C1191-201. doi: 10.1152/ajpcell.00144.2008. Epub 2008 Sep 3. Am J Physiol Cell Physiol. 2008. PMID: 18768927 Free PMC article.
-
A rat parotid gland cell line, Par-C10, exhibits neurotransmitter-regulated transepithelial anion secretion.Am J Physiol. 1998 Aug;275(2):C367-74. doi: 10.1152/ajpcell.1998.275.2.C367. Am J Physiol. 1998. PMID: 9688590
-
Salivary gland cell differentiation and organization on micropatterned PLGA nanofiber craters.Biomaterials. 2013 Sep;34(28):6773-84. doi: 10.1016/j.biomaterials.2013.05.061. Epub 2013 Jun 15. Biomaterials. 2013. PMID: 23777914 Free PMC article.
-
Functional spheroid organization of human salivary gland cells cultured on hydrogel-micropatterned nanofibrous microwells.Acta Biomater. 2016 Nov;45:121-132. doi: 10.1016/j.actbio.2016.08.058. Epub 2016 Sep 1. Acta Biomater. 2016. PMID: 27592814
-
Disruption of tight junction structure in salivary glands from Sjögren's syndrome patients is linked to proinflammatory cytokine exposure.Arthritis Rheum. 2010 May;62(5):1280-9. doi: 10.1002/art.27362. Arthritis Rheum. 2010. PMID: 20131287
Cited by
-
Implantable three-dimensional salivary spheroid assemblies demonstrate fluid and protein secretory responses to neurotransmitters.Tissue Eng Part A. 2013 Jul;19(13-14):1610-20. doi: 10.1089/ten.TEA.2012.0301. Epub 2013 May 10. Tissue Eng Part A. 2013. PMID: 23442148 Free PMC article.
-
Slow hydrogel matrix degradation enhances salivary gland mimetic phenotype.Acta Biomater. 2023 Aug;166:187-200. doi: 10.1016/j.actbio.2023.05.005. Epub 2023 May 5. Acta Biomater. 2023. PMID: 37150277 Free PMC article.
-
Bioprinting salivary gland models and their regenerative applications.BDJ Open. 2024 May 30;10(1):39. doi: 10.1038/s41405-024-00219-2. BDJ Open. 2024. PMID: 38816372 Free PMC article. Review.
-
Hydrogels for Salivary Gland Tissue Engineering.Gels. 2022 Nov 10;8(11):730. doi: 10.3390/gels8110730. Gels. 2022. PMID: 36354638 Free PMC article. Review.
-
Strategies for Developing Functional Secretory Epithelia from Porcine Salivary Gland Explant Outgrowth Culture Models.Biomolecules. 2019 Oct 25;9(11):657. doi: 10.3390/biom9110657. Biomolecules. 2019. PMID: 31717706 Free PMC article.
References
-
- Melvin J.E. Yule D. Shuttleworth T. Begenisich T. Regulation of fluid and electrolyte secretion in salivary gland acinar cells. Annu Rev Physiol. 2005;67:445. - PubMed
-
- Martinez J.R. Cassity N. Salivary secretion induced from isolated, perfused rat submandibular glands by sympathomimetic agents. Arch Oral Biol. 1983;28:1101. - PubMed
-
- Martinez J.R. Holzgreve H. Frick A. Micropuncture study of submaxillary glands of adult rats. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;290:124. - PubMed
-
- Nusrat A. Turner J.R. Madara J.L. Molecular physiology and pathophysiology of tight junctions. IV. Regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am J Physiol Gastrointest Liver Physiol. 2000;279:G851. - PubMed
-
- Shin K. Fogg V.C. Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

