Small interference RNA profiling reveals the essential role of human membrane trafficking genes in mediating the infectious entry of dengue virus
- PMID: 20122152
- PMCID: PMC2825209
- DOI: 10.1186/1743-422X-7-24
Small interference RNA profiling reveals the essential role of human membrane trafficking genes in mediating the infectious entry of dengue virus
Abstract
Background: Dengue virus (DENV) is the causative agent of Dengue fever and the life-threatening Dengue Haemorrhagic fever or Dengue shock syndrome. In the absence of anti-viral agents or vaccine, there is an urgent need to develop an effective anti-viral strategy against this medically important viral pathogen. The initial interplay between DENV and the host cells may represent one of the potential anti-viral targeting sites. Currently the involvements of human membrane trafficking host genes or factors that mediate the infectious cellular entry of dengue virus are not well defined.
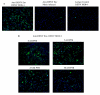
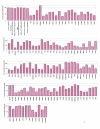
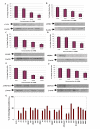
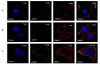
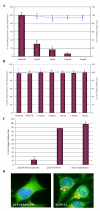
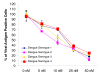
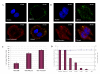
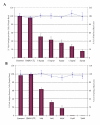
Results: In this study, we have used a targeted small interfering RNA (siRNA) library to identify and profile key cellular genes involved in processes of endocytosis, cytoskeletal dynamics and endosome trafficking that are important and essential for DENV infection. The infectious entry of DENV into Huh7 cells was shown to be potently inhibited by siRNAs targeting genes associated with clathrin-mediated endocytosis. The important role of clathrin-mediated endocytosis was confirmed by the expression of well-characterized dominant-negative mutants of genes in this pathway and by using the clathrin endocytosis inhibitor chlorpromazine. Furthermore, DENV infection was shown to be sensitive to the disruption of human genes in regulating the early to late endosomal trafficking as well as the endosomal acidic pH. The importance and involvement of both actin and microtubule dynamics in mediating the infectious entry of DENV was also revealed in this study.
Conclusions: Together, the findings from this study have provided a detail profiling of the human membrane trafficking cellular genes and the mechanistic insight into the interplay of these host genes with DENV to initiate an infection, hence broadening our understanding on the entry pathway of this medically important viral pathogen. These data may also provide a new potential avenue for development of anti-viral strategies and treatment of DENV infection.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

