Trastuzumab with either docetaxel or vinorelbine as first-line treatment for patients with HER2-positive advanced breast cancer: a retrospective comparison
- PMID: 20122160
- PMCID: PMC2835645
- DOI: 10.1186/1471-2407-10-28
Trastuzumab with either docetaxel or vinorelbine as first-line treatment for patients with HER2-positive advanced breast cancer: a retrospective comparison
Abstract
Background: Combinations of trastuzumab with either docetaxel or vinorelbine are considered valuable treatment options for HER2-positive metastatic breast cancer patients. We performed a retrospective comparison of the clinical outcomes associated with either one of these combinations.
Methods: From a multi-institutional database we retrieved 179 patients treated with either docetaxel or vinorelbine plus trastuzumab as first-line therapy for HER2-positive advanced breast cancer.
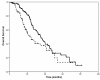
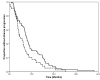
Results: Docetaxel-trastuzumab was superior to vinorelbine-trastuzumab in terms of response rate (RR: 77 vs 57%, p = 0.01) and median overall survival (OS: 35 vs 23 months, p = 0.04), but not in median time to progression (TTP: 12 vs 10 months, p = 0.53). At multivariate analysis, type of treatment was not associated with TTP but was an independent predictor of OS, with a significant reduction in the risk of death in favor of docetaxel-trastuzumab (HR 0.474, 95% IC 0,303-0.742, p < 0.01).
Conclusion: Docetaxel or vinorelbine, when combined with trastuzumab, provide excellent rates of tumor control in patients with previously untreated HER2-positive advanced breast cancer. Docetaxel may offer some advantage in terms of response rate and resulted in a significantly prolonged overall survival, which, because of the retrospective design of our study, deserves further investigation in prospective trials.
Figures

References
-
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. - DOI - PubMed
-
- Marty M, Cognetti F, Maraninchi D, Snyder R, Mauriac L, Tubiana-Hulin M, Chan S, Grimes D, Anton A, Lluch A, Kennedy J, O'Byrne K, Conte P, Green M, Ward C, Mayne K, Extra JM. Randomized phase II trial of the efficacy and safety of trastuzumab combined with docetaxel in patients with human epidermal growth factor receptor 2-positive metastatic breast cancer administered as first-line treatment: the M77001 study group. J Clin Oncol. 2005;23:4265–4274. doi: 10.1200/JCO.2005.04.173. - DOI - PubMed
-
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. - DOI - PubMed
-
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr, Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. - DOI - PubMed
-
- Pegram MD, Konecny GE, O'Callaghan C, Beryt M, Pietras R, Slamon DJ. Rational combinations of trastuzumab with chemotherapeutic drugs used in the treatment of breast cancer. J Natl Cancer Inst. 2004;96:739–749. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

