Docosahexaenoic acid prevents dendritic cell maturation and in vitro and in vivo expression of the IL-12 cytokine family
- PMID: 20122166
- PMCID: PMC2827414
- DOI: 10.1186/1476-511X-9-12
Docosahexaenoic acid prevents dendritic cell maturation and in vitro and in vivo expression of the IL-12 cytokine family
Abstract
Background: Acute and chronic inflammation play essential roles in inflammatory/autoimmune conditions. Protective anti-inflammatory effects of the n-3 fatty acids docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) were reported in animal models of colitis, sepsis, and stroke. Since dendritic cells (DC) represent the essential cellular link between innate and adaptive immunity and have a prominent role in tolerance for self-antigens, we sought to investigate the impact of DHA on DC maturation and proinflammatory cytokine production.
Methods: Murine bone marrow-derived DC were treated with DHA and stimulated with various toll-like receptor (TLR) ligands. Flow cytometry was used to determine the levels of surface maturation markers and endocytic activity. Cytokine expression and secretion were measured by real-time RT-PCR and ELISA assays. PPARgamma and NFkappaB activity in nuclear extracts were determined by binding to specific oligonucleotide sequences using ELISA-based assays. In vivo effects of DHA were assessed in splenic DC from LPS-inoculated mice maintained on a DHA-enriched diet.
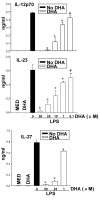
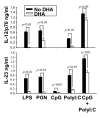
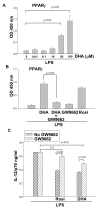
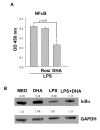
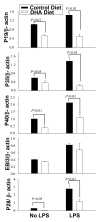
Results: DHA maintained the immature phenotype in bone marrow-derived DC by preventing the upregulation of MHCII and costimulatory molecules (CD40, CD80 and CD86) and maintaining high levels of endocytic activity. DHA inhibited the production of pro-inflammatory cytokines, including the IL-12 cytokine family (IL-12p70, IL-23, and IL-27), from DC stimulated with TLR2, 3, 4, and 9 ligands. DHA inhibition of IL-12 expression was mediated through activation of PPARgamma and inhibition of NFkappaBp65 nuclear translocation. DHA exerted a similar inhibitory effect on IL-12 and IL-23 expression in vivo in LPS-inoculated mice maintained on a DHA-enriched diet.
Conclusions: Exposure of bone marrow-derived DC to DHA resulted in the maintenance of an immature phenotype and drastic reduction in proinflammatory cytokine release. DHA inhibited the expression and secretion of the IL-12 cytokine family members (IL-12p70, IL-23 and IL-27), which play essential roles in the differentiation of the proinflammatory Th1/Th17 effector cells. The effect of DHA on IL-12 expression was mediated through activation of PPARgamma and inhibition of NFkappaB. Inhibition of IL-12 and IL-23 expression was also evident in splenic DC from mice fed a DHA-enriched diet, suggesting that dietary DHA acts as an anti-inflammatory agent in vivo.
Figures


References
-
- Orr SK, Bazinet RP. The emerging role of docosahexaenoic acid in neuroinflammation. Curr Opin Investig Drugs. 2008;9(7):735–743. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

