Pentastatin-1, a collagen IV derived 20-mer peptide, suppresses tumor growth in a small cell lung cancer xenograft model
- PMID: 20122172
- PMCID: PMC2824711
- DOI: 10.1186/1471-2407-10-29
Pentastatin-1, a collagen IV derived 20-mer peptide, suppresses tumor growth in a small cell lung cancer xenograft model
Abstract
Background: Angiogenesis is the formation of neovasculature from a pre-existing vascular network. Progression of solid tumors including lung cancer is angiogenesis-dependent. We previously introduced a bioinformatics-based methodology to identify endogenous anti-angiogenic peptide sequences, and validated these predictions in vitro in human umbilical vein endothelial cell (HUVEC) proliferation and migration assays.
Methods: One family of peptides with high activity is derived from the alpha-fibrils of type IV collagen. Based on the results from the in vitro screening, we have evaluated the ability of a 20 amino acid peptide derived from the alpha5 fibril of type IV collagen, pentastatin-1, to suppress vessel growth in an angioreactor-based directed in vivo angiogenesis assay (DIVAA). In addition, pentastatin-1 suppressed tumor growth with intraperitoneal peptide administration in a small cell lung cancer (SCLC) xenograft model in nude mice using the NCI-H82 human cancer cell line.
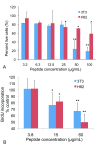
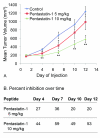
Results: Pentastatin-1 decreased the invasion of vessels into angioreactors in vivo in a dose dependent manner. The peptide also decreased the rate of tumor growth and microvascular density in vivo in a small cell lung cancer xenograft model.
Conclusions: The peptide treatment significantly decreased the invasion of microvessels in angioreactors and the rate of tumor growth in the xenograft model, indicating potential treatment for angiogenesis-dependent disease, and for translational development as a therapeutic agent for lung cancer.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

