Intracellular Ca2+ regulating proteins in vascular smooth muscle cells are altered with type 1 diabetes due to the direct effects of hyperglycemia
- PMID: 20122173
- PMCID: PMC2829469
- DOI: 10.1186/1475-2840-9-8
Intracellular Ca2+ regulating proteins in vascular smooth muscle cells are altered with type 1 diabetes due to the direct effects of hyperglycemia
Abstract
Background: Diminished calcium (Ca2+) transients in response to physiological agonists have been reported in vascular smooth muscle cells (VSMCs) from diabetic animals. However, the mechanism responsible was unclear.
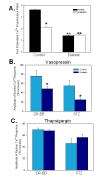
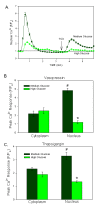
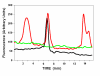
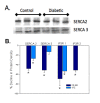
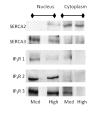
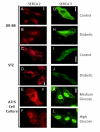
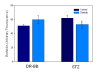
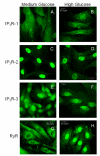
Methodology/principal findings: VSMCs from autoimmune type 1 Diabetes Resistant Bio-Breeding (DR-BB) rats and streptozotocin-induced rats were examined for levels and distribution of inositol trisphosphate receptors (IP3R) and the SR Ca2+ pumps (SERCA 2 and 3). Generally, a decrease in IP3R levels and dramatic increase in ryanodine receptor (RyR) levels were noted in the aortic samples from diabetic animals. Redistribution of the specific IP3R subtypes was dependent on the rat model. SERCA 2 was redistributed to a peri-nuclear pattern that was more prominent in the DR-BB diabetic rat aorta than the STZ diabetic rat. The free intracellular Ca2+ in freshly dispersed VSMCs from control and diabetic animals was monitored using ratiometric Ca2+ sensitive fluorophores viewed by confocal microscopy. In control VSMCs, basal fluorescence levels were significantly higher in the nucleus relative to the cytoplasm, while in diabetic VSMCs they were essentially the same. Vasopressin induced a predictable increase in free intracellular Ca2+ in the VSMCs from control rats with a prolonged and significantly blunted response in the diabetic VSMCs. A slow rise in free intracellular Ca2+ in response to thapsigargin, a specific blocker of SERCA was seen in the control VSMCs but was significantly delayed and prolonged in cells from diabetic rats. To determine whether the changes were due to the direct effects of hyperglycemica, experiments were repeated using cultured rat aortic smooth muscle cells (A7r5) grown in hyperglycemic and control conditions. In general, they demonstrated the same changes in protein levels and distribution as well as the blunted Ca2+ responses to vasopressin and thapsigargin as noted in the cells from diabetic animals.
Conclusions/significance: This work demonstrates that the previously-reported reduced Ca2+ signaling in VSMCs from diabetic animals is related to decreases and/or redistribution in the IP3R Ca2+ channels and SERCA proteins. These changes can be duplicated in culture with high glucose levels.
Figures


References
-
- Preis S, Hwang S, Coady S, Pencina M, D'Agostino RS, Savage P, Levy D, Fox C. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the Framingham Heart Study, 1950 to 2005. Circulation. 2009;119:1728–1735. doi: 10.1161/CIRCULATIONAHA.108.829176. - DOI - PMC - PubMed
-
- Aronson D. Hyperglycemia and the pathobiology of diabetic complications. Adv Cardiol. 2008;45:1–16. full_text. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

