Antimicrobial peptaibols, novel suppressors of tumor cells, targeted calcium-mediated apoptosis and autophagy in human hepatocellular carcinoma cells
- PMID: 20122248
- PMCID: PMC2825246
- DOI: 10.1186/1476-4598-9-26
Antimicrobial peptaibols, novel suppressors of tumor cells, targeted calcium-mediated apoptosis and autophagy in human hepatocellular carcinoma cells
Abstract
Background: Hepatocellular carcinoma (HCC) is one of the most common cancers in the world which is highly chemoresistant to currently available chemotherapeutic agents. Thus, novel therapeutic targets are needed to be sought for the successful treatment of HCC. Peptaibols, a family of peptides synthesized non-ribosomally by the Trichoderma species and other fungi, exhibit antibiotic activities against bacteria and fungi. Few studies recently showed that peptaibols exerted cytotoxicity toward human lung epithelial and breast carcinoma cells. However, the mechanism involved in peptaibol-induced cell death remains poorly understood.
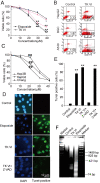
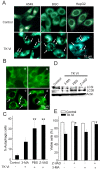
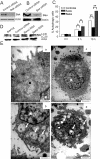
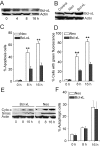
Results: Here, we showed that Trichokonin VI (TK VI), a peptaibol from Trichoderma pseudokoningii SMF2, induced growth inhibition of HCC cells in a dose-dependent manner. It did not obviously impair the viability of normal liver cells at lower concentration. Moreover, the suppression of cell viability resulted from the programmed cell death (PCD) with characteristics of apoptosis and autophagy. An influx of Ca2+ triggered the activation of mu-calpain and proceeded to the translocation of Bax to mitochondria and subsequent promotion of apoptosis. On the other hand, typically morphological characteristics consistent with autophagy were also observed by punctate distribution of MDC staining and the induction of LC3-II, including extensive autophagic vacuolization and enclosure of cell organelles by these autophagosomes. More significantly, specific depletion of Bak expression by small RNA interfering (siRNA) could partly attenuate TK VI-induced autophagy. However, siRNA against Bax led to increased autophagy.
Conclusion: Taken together, these findings showed for the first time that peptaibols were novel regulators involved in both apoptosis and autophagy, suggesting that the class of peptaibols might serve as potential suppressors of tumor cells.
Figures




Similar articles
-
Calpain, Atg5 and Bak play important roles in the crosstalk between apoptosis and autophagy induced by influx of extracellular calcium.Apoptosis. 2013 Apr;18(4):435-51. doi: 10.1007/s10495-012-0786-2. Apoptosis. 2013. PMID: 23242420
-
Antimicrobial peptaibols from Trichoderma pseudokoningii induce programmed cell death in plant fungal pathogens.Microbiology (Reading). 2012 Jan;158(Pt 1):166-175. doi: 10.1099/mic.0.052670-0. Epub 2011 Nov 3. Microbiology (Reading). 2012. PMID: 22053006
-
Cellular and molecular insight into the inhibition of primary root growth of Arabidopsis induced by peptaibols, a class of linear peptide antibiotics mainly produced by Trichoderma spp.J Exp Bot. 2016 Apr;67(8):2191-205. doi: 10.1093/jxb/erw023. Epub 2016 Feb 5. J Exp Bot. 2016. PMID: 26850879 Free PMC article.
-
The involvement of ROS-regulated programmed cell death in hepatocellular carcinoma.Crit Rev Oncol Hematol. 2024 May;197:104361. doi: 10.1016/j.critrevonc.2024.104361. Epub 2024 Apr 16. Crit Rev Oncol Hematol. 2024. PMID: 38626849 Review.
-
Tolypocladamides A-G: Cytotoxic Peptaibols from Tolypocladium inflatum.J Nat Prod. 2022 Jun 24;85(6):1603-1616. doi: 10.1021/acs.jnatprod.2c00240. Epub 2022 Jun 13. J Nat Prod. 2022. PMID: 35696348 Free PMC article. Review.
Cited by
-
Peptaibol-Containing Extracts of Trichoderma atroviride and the Fight against Resistant Microorganisms and Cancer Cells.Molecules. 2021 Oct 4;26(19):6025. doi: 10.3390/molecules26196025. Molecules. 2021. PMID: 34641569 Free PMC article.
-
Water-Soluble Trichogin GA IV-Derived Peptaibols Protect Tomato Plants From Botrytis cinerea Infection With Limited Impact on Plant Defenses.Front Plant Sci. 2022 May 19;13:881961. doi: 10.3389/fpls.2022.881961. eCollection 2022. Front Plant Sci. 2022. PMID: 35665189 Free PMC article.
-
Synergistic combination of alkylphosphocholines with peptaibols in targeting Leishmania infantum in vitro.Int J Parasitol Drugs Drug Resist. 2018 Aug;8(2):194-202. doi: 10.1016/j.ijpddr.2018.03.005. Epub 2018 Mar 20. Int J Parasitol Drugs Drug Resist. 2018. PMID: 29631127 Free PMC article.
-
Fungal Guttation, a Source of Bioactive Compounds, and Its Ecological Role-A Review.Biomolecules. 2021 Aug 25;11(9):1270. doi: 10.3390/biom11091270. Biomolecules. 2021. PMID: 34572483 Free PMC article. Review.
-
14-Residue peptaibol velutibol A from Trichoderma velutinum: its structural and cytotoxic evaluation.RSC Adv. 2020 Aug 24;10(52):31233-31242. doi: 10.1039/d0ra05780k. eCollection 2020 Aug 21. RSC Adv. 2020. PMID: 35520634 Free PMC article.
References
-
- Schepelmann S, Hallenbeck P, Ogilvie LM, Hedley D, Friedlos F, Martin J, Scanlon I, Hay C, Hawkins LK, Marais R, Springer CJ. Systemic gene-directed enzyme prodrug therapy of hepatocellular carcinoma using a targeted adenovirus armed with carboxypeptidase G2. Cancer Res. 2005;65:5003–8. doi: 10.1158/0008-5472.CAN-05-0393. - DOI - PubMed
-
- Wang SW, Pan SL, Huang YC, Guh JH, Chiang PC, Huang DY, Kuo SC, Lee KH, Teng CM. CHM-1, a novel synthetic quinolone with potent and selective antimitotic antitumor activity against human hepatocellular carcinoma in vitro and in vivo. Mol Cancer Ther. 2008;7:350–60. doi: 10.1158/1535-7163.MCT-07-2000. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

