Adherens junctions connect stress fibres between adjacent endothelial cells
- PMID: 20122254
- PMCID: PMC2845098
- DOI: 10.1186/1741-7007-8-11
Adherens junctions connect stress fibres between adjacent endothelial cells
Abstract
Background: Endothelial cell-cell junctions maintain endothelial integrity and regulate vascular morphogenesis and homeostasis. Cell-cell junctions are usually depicted with a linear morphology along the boundaries between adjacent cells and in contact with cortical F-actin. However, in the endothelium, cell-cell junctions are highly dynamic and morphologically heterogeneous.
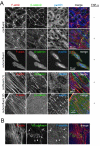
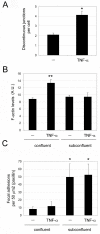
Results: We report that endothelial cell-cell junctions can attach to the ends of stress fibres instead of to cortical F-actin, forming structures that we name discontinuous adherens junctions (AJ). Discontinuous AJ are highly dynamic and are increased in response to tumour necrosis factor (TNF)-alpha, correlating with the appearance of stress fibres. We show that vascular endothelial (VE)-cadherin/beta-catenin/alpha-catenin complexes in discontinuous AJ are linked to stress fibres. Moreover, discontinuous AJ connect stress fibres from adjacent cells independently of focal adhesions, of which there are very few in confluent endothelial cells, even in TNF-alpha-stimulated cells. RNAi-mediated knockdown of VE-cadherin, but not zonula occludens-1, reduces the linkage of stress fibres to cell-cell junctions, increases focal adhesions, and dramatically alters the distribution of these actin cables in confluent endothelial cells.
Conclusions: Our results indicate that stress fibres from neighbouring cells are physically connected through discontinuous AJ, and that stress fibres can be stabilized by AJ-associated multi-protein complexes distinct from focal adhesions.
Figures







Comment in
-
Adherens junctions remain dynamic.BMC Biol. 2010 Apr 8;8:34. doi: 10.1186/1741-7007-8-34. BMC Biol. 2010. PMID: 20377885 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

