Evaluation of an in-clinic Serum Amyloid A (SAA) assay and assessment of the effects of storage on SAA samples
- PMID: 20122257
- PMCID: PMC2831015
- DOI: 10.1186/1751-0147-52-8
Evaluation of an in-clinic Serum Amyloid A (SAA) assay and assessment of the effects of storage on SAA samples
Abstract
Background: An in-clinic assay for equine serum amyloid A (SAA) analysis, Equinostic EVA1, was evaluated for use in a clinical setting. Stability of SAA in serum samples was determined.
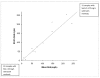
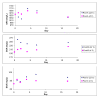
Methods: Intra- and inter- assay variation of the in-clinic method was determined. The in-clinic method (EVA1) results were compared to a reference method (Eiken LZ SAA) with 62 patient samples. For samples with SAA concentrations within the assay range of EVA1 (10-270 mg/L), differences between the methods were evaluated in a difference plot. Linearity under dilution was evaluated in two samples. Stability of SAA in three serum pools stored at 4 degrees C and approximately 22 degrees C was evaluated with the reference method day 0, 1, 2, 4, 7, 17 and analysed with a two-way ANOVA.
Results: The imprecision (coefficient of variation, CV) for the in-clinic method was acceptable at higher SAA concentrations with CV values of 7,3-12%, but poor at low SAA concentrations with CV values of 27% and 37% for intra- and inter-assay variation respectively. Recovery after dilution was 50-138%. The in-clinic assay and the reference method identified equally well horses with low (<10 mg/L) and high (>270 mg/L) SAA concentrations. Within the assay range of the in-clinic method, 10-270 mg/L, the difference between the two methods was slightly higher than could be explained by the inherent imprecision of the assays. There were no significant changes of serum SAA concentrations during storage.
Conclusions: The in-clinic assay identified horses with SAA concentrations of <10 mg/L and >270 mg/L in a similar way as the reference method, and provided an estimate of the SAA concentration in the range of 10-270 mg/L. The imprecision of the in-clinic method was acceptable at high SAA concentrations but not at low concentrations. Dilution of samples gave inconsistent results. SAA was stable both at room temperature and refrigerated, and thus samples may be stored before analysis with the reference method.
Figures

References
-
- Pepys MB, Baltz ML, Tennent GA, Kent J, Ousey J, Rossdale PD. Serum amyloid A protein (SAA) in horses: objective measurement of the acute phase response. Equine Vet J. 1989;21(2):106–109. - PubMed
-
- Nunokawa Y, Fujinaga T, Taira T, Okumura M, Yamashita K, Tsunoda N, Hagio M. Evaluation of serum amyloid A protein as an acute-phase reactive protein in horses. J Vet Med Sci. 1993;55(6):1011–1016. - PubMed
-
- Hulten C, Tulamo RM, Suominen MM, Burvall K, Marhaug G, Forsberg M. A non-competitive chemiluminescence enzyme immunoassay for the equine acute phase protein serum amyloid A (SAA) -- a clinically useful inflammatory marker in the horse. Vet Immunol Immunopathol. 1999;68(2-4):267–281. doi: 10.1016/S0165-2427(99)00027-6. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

