Do pneumococcal conjugate vaccines provide any cross-protection against serotype 19A?
- PMID: 20122261
- PMCID: PMC2829470
- DOI: 10.1186/1471-2431-10-4
Do pneumococcal conjugate vaccines provide any cross-protection against serotype 19A?
Abstract
Background: Introduction of the 7-valent pneumococcal conjugate vaccine (7vCRM) in several countries has led to a rapid, significant drop in vaccine-type invasive pneumococcal disease (IPD) in immunized children. In the United States and some other countries with high antibiotic use, a subsequent rise in serotype 19A IPD has been taken to indicate that the 19F conjugate in the vaccine provides no cross-protection against the immunologically related 19A.
Discussion: We systematically assessed the clinical efficacy and effectiveness of 19F-containing vaccines against 19A disease or nasopharyngeal carriage by searching English-language articles in the electronic databases PubMed, Current contents, Scopus, and Embase from 1985 to 2008. The vaccine efficacy and effectiveness point estimates were consistently positive for modest protection against 19A IPD and acute otitis media (AOM). However, statistical significance was not reached in any individual study. No consistent impact of 7vCRM on 19A nasopharyngeal colonization could be detected. These findings are discussed in context of immunogenicity analyses indicating that 7vCRM induces functionally active anti-19A antibodies after the booster dose, and that other 19F-containing vaccine formulations may elicit higher levels of such antibodies after both primary and booster doses.
Summary: Taken together, these results suggest that 19F-conjugates can provide some protection against 19A disease. The magnitude of this protection in a given setting will likely depend on several factors. These include the anti-19A immunogenicity of the specific vaccine formulation, the number of doses of that formulation needed to elicit the response, and the burden of 19A disease that occurs after those doses. It is possible that a modest protective effect may be obscured by the presence of countervailing selection pressures (such as high antibiotic use) that favor an increase in colonization with antibiotic-non-susceptible strains of 19A.
Figures
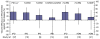
References
-
- Pelton SI, Huot H, Finkelstein JA, Bishop CJ, Hsu KK, Kellenberg J, Huang SS, Goldstein R, Hanage WP. Emergence of 19A as virulent and multidrug resistant Pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2007;26(6):468–472. doi: 10.1097/INF.0b013e31803df9ca. - DOI - PubMed
-
- Singleton RJ, Hennessy TW, Bulkow LR, Hammitt LL, Zulz T, Hurlburt DA, Butler JC, Rudolph K, Parkinson A. Invasive pneumococcal disease caused by nonvaccine serotypes among alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. JAMA. 2007;297(16):1784–1792. doi: 10.1001/jama.297.16.1784. - DOI - PubMed
-
- Hicks LA, Harrison LH, Flannery B, Hadler JL, Schaffner W, Craig AS, Jackson D, Thomas A, Beall B, Lynfield R. Incidence of pneumococcal disease due to non-pneumococcal conjugate vaccine (PCV7) serotypes in the United States during the era of widespread PCV7 vaccination, 1998-2004. J Infect Dis. 2007;196(9):1346–1354. doi: 10.1086/521626. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

