The effects of pulmonary rehabilitation in patients with non-cystic fibrosis bronchiectasis: protocol for a randomised controlled trial
- PMID: 20122281
- PMCID: PMC2845119
- DOI: 10.1186/1471-2466-10-5
The effects of pulmonary rehabilitation in patients with non-cystic fibrosis bronchiectasis: protocol for a randomised controlled trial
Abstract
Background: Non-cystic fibrosis bronchiectasis is characterised by sputum production, exercise limitation and recurrent infections. Although pulmonary rehabilitation is advocated for this patient group, its effects are unclear. The aims of this study are to determine the short and long term effects of pulmonary rehabilitation on exercise capacity, cough, quality of life and the incidence of acute pulmonary exacerbations.
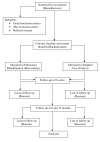
Methods/design: This randomised controlled trial aims to recruit 64 patients with bronchiectasis from three tertiary institutions. Participants will be randomly allocated to the intervention group (supervised, twice weekly exercise training with regular review of airway clearance therapy) or a control group (twice weekly telephone support). Measurements will be taken at baseline, immediately following the intervention and at six and 12 months following the intervention period by a blinded assessor. Exercise capacity will be measured using the incremental shuttle walk test and the six-minute walk test. Quality of life and health status will be measured using the Chronic Respiratory Questionnaire, Leicester Cough Questionnaire, Assessment of Quality of Life Questionnaire and the Hospital Anxiety and Depression Scale. The rate of hospitalisation will be captured as well as the incidence of acute pulmonary exacerbations using a daily symptom diary.
Discussion: Results from this study will help to determine the efficacy of supervised twice-weekly pulmonary rehabilitation upon exercise capacity and quality of life in patients with bronchiectasis and will contribute to clinical practice guidelines for physiotherapists in the management of this population.
Trial registration: This study protocol is registered with ClinicalTrials.gov (NCT00885521).
Figures
References
-
- Wilson C, Jones P, O'Leary CJ, Cole P, Wilson R. Validation of the St George's Respiratory Questionnaire in Bronchiectasis. Am J Respir Crit Care Med. 1997;156:536–541. - PubMed
-
- Koulouris N, Retsou S, Kosmas E, Dimakou K, Malagari K, Mantzikopoulos G, Koutsoukou A, Milic-Emili J, Jordanoglou J. Tidal expiratory flow limitation, dyspnoea and exercise capacity in patients with bilateral bronchiectasis. Eur Respir J. 2003;21:743–748. doi: 10.1183/09031936.03.00301103. - DOI - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical