The role of ABCE1 in eukaryotic posttermination ribosomal recycling
- PMID: 20122402
- PMCID: PMC2951834
- DOI: 10.1016/j.molcel.2009.12.034
The role of ABCE1 in eukaryotic posttermination ribosomal recycling
Abstract
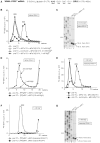
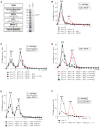
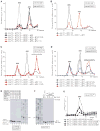
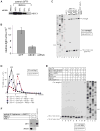
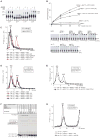
After termination, eukaryotic 80S ribosomes remain associated with mRNA, P-site deacylated tRNA, and release factor eRF1 and must be recycled by dissociating these ligands and separating ribosomes into subunits. Although recycling of eukaryotic posttermination complexes (post-TCs) can be mediated by initiation factors eIF3, eIF1, and eIF1A (Pisarev et al., 2007), this energy-free mechanism can function only in a narrow range of low Mg(2+) concentrations. Here, we report that ABCE1, a conserved and essential member of the ATP-binding cassette (ABC) family of proteins, promotes eukaryotic ribosomal recycling over a wide range of Mg(2+) concentrations. ABCE1 dissociates post-TCs into free 60S subunits and mRNA- and tRNA-bound 40S subunits. It can hydrolyze ATP, GTP, UTP, and CTP. NTP hydrolysis by ABCE1 is stimulated by post-TCs and is required for its recycling activity. Importantly, ABCE1 dissociates only post-TCs obtained with eRF1/eRF3 (or eRF1 alone), but not post-TCs obtained with puromycin in eRF1's absence.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures

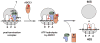
References
-
- Alkalaeva EZ, Pisarev AV, Frolova LY, Kisselev LL, Pestova TV. In vitro reconstitution of eukaryotic translation reveals cooperativity between release factors eRF1 and eRF3. Cell. 2006;125:1125–1136. - PubMed
-
- Andersen CB, Becker T, Blau M, Anand M, Halic M, Balar B, Mielke T, Boesen T, Pedersen JS, Spahn CM, et al. Structure of eEF3 and the mechanism of transfer RNA release from the E-site. Nature. 2006;443:663–668. - PubMed
-
- Andersen DS, Leevers SJ. The essential Drosophila ATP-binding cassette domain protein, pixie, binds the 40 S ribosome in an ATP-dependent manner and is required for translation initiation. J Biol Chem. 2007;282:14752–14760. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

