Comparative analysis of MVA-CD40L and MVA-TRICOM vectors for enhancing the immunogenicity of chronic lymphocytic leukemia (CLL) cells
- PMID: 20122733
- PMCID: PMC2891581
- DOI: 10.1016/j.leukres.2009.12.013
Comparative analysis of MVA-CD40L and MVA-TRICOM vectors for enhancing the immunogenicity of chronic lymphocytic leukemia (CLL) cells
Abstract
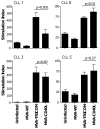
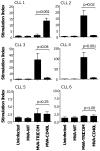
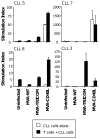
Adenoviral transduction with CD40L and poxviral transduction with B7-1, ICAM-1, and LFA-3 (TRICOM) have been used to enhance the antigen-presenting capacity of chronic lymphocytic leukemia (CLL) cells. This study compares the same vector (modified vaccinia virus strain Ankara (MVA)) encoding CD40L or TRICOM for its ability to enhance the immunogenicity of CLL cells. CLL cells from some patients showed differential responses to each vector in terms of induction of autologous T-cell responses. This study supports the rationale for the use of CLL cells modified ex vivo with pre-specified recombinant MVA vectors as a whole tumor-cell vaccine for immunotherapy in CLL patients.
Published by Elsevier Ltd.
Figures

References
-
- Wierda WG, Kipps TJ. Chronic lymphocytic leukemia. Curr Opin Hematol. 1999;6:253–61. - PubMed
-
- Tsimberidou AM, Keating MJ. Treatment of fludarabine-refractory chronic lymphocytic leukemia. Cancer. 2009;115:2824–36. - PubMed
-
- Pleyer L, Egle A, Hartmann TN, Greil R. Molecular and cellular mechanisms of CLL: novel therapeutic approaches. Nat Rev Clin Oncol. 2009;6:405–18. Epub 2009 Jun 2. - PubMed
-
- Buhmann R, Nolte A, Westhaus D, Emmerich B, Hallek M. CD40-activated B-cell chronic lymphocytic leukemia cells for tumor immunotherapy: stimulation of allogeneic versus autologous T cells generates different types of effector cells. Blood. 1999;93:1992–2002. - PubMed
-
- Rezvany MR, Jeddi-Tehrani M, Rabbani H, Lewin N, Avila-Carino J, Osterborg A, et al. Autologous T lymphocytes may specifically recognize leukaemic B cells in patients with chronic lymphocytic leukaemia. Br J Haematol. 2000;111:608–17. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

