Tuba1a gene expression is regulated by KLF6/7 and is necessary for CNS development and regeneration in zebrafish
- PMID: 20123021
- PMCID: PMC2837137
- DOI: 10.1016/j.mcn.2010.01.004
Tuba1a gene expression is regulated by KLF6/7 and is necessary for CNS development and regeneration in zebrafish
Abstract
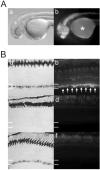
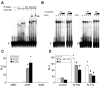
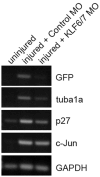
We report that knockdown of the alpha1 tubulin isoform Tuba1a, but not the highly related Tuba1b, dramatically impedes nervous system formation during development and RGC axon regeneration following optic nerve injury in adults. Within the tuba1a promoter, a G/C-rich element was identified that is necessary for tuba1a induction during RGC differentiation and optic axon regeneration. KLF6a and 7a, which we previously reported are essential for optic axon regeneration (Veldman et al., 2007), bind this G/C-rich element and transactivate the tuba1a promoter. In vivo knockdown of KLF6a and 7a attenuate regeneration-dependent activation of the endogenous tuba1a and p27 genes. These results suggest tuba1a expression is necessary for CNS development and regeneration and that KLF6a and 7a mediate their effects, at least in part, via transcriptional control of tuba1a promoter activity.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Gene expression analysis of zebrafish retinal ganglion cells during optic nerve regeneration identifies KLF6a and KLF7a as important regulators of axon regeneration.Dev Biol. 2007 Dec 15;312(2):596-612. doi: 10.1016/j.ydbio.2007.09.019. Epub 2007 Sep 22. Dev Biol. 2007. PMID: 17949705
-
An element in the alpha1-tubulin promoter is necessary for retinal expression during optic nerve regeneration but not after eye injury in the adult zebrafish.J Neurosci. 2004 Sep 1;24(35):7663-73. doi: 10.1523/JNEUROSCI.2281-04.2004. J Neurosci. 2004. PMID: 15342733 Free PMC article.
-
Expression of SoxC Transcription Factors during Zebrafish Retinal and Optic Nerve Regeneration.Neurosci Bull. 2017 Feb;33(1):53-61. doi: 10.1007/s12264-016-0073-2. Epub 2016 Oct 14. Neurosci Bull. 2017. PMID: 27743342 Free PMC article.
-
Lab review: Molecular dissection of the signal transduction pathways associated with PTEN deletion-induced optic nerve regeneration.Restor Neurol Neurosci. 2019;37(6):545-552. doi: 10.3233/RNN-190949. Restor Neurol Neurosci. 2019. PMID: 31839616 Free PMC article. Review.
-
Regeneration of axons in the visual system.Restor Neurol Neurosci. 2008;26(2-3):147-74. Restor Neurol Neurosci. 2008. PMID: 18820408 Review.
Cited by
-
Genetic dissection of axon regeneration.Curr Opin Neurobiol. 2011 Feb;21(1):189-96. doi: 10.1016/j.conb.2010.08.010. Epub 2010 Sep 9. Curr Opin Neurobiol. 2011. PMID: 20832288 Free PMC article. Review.
-
Dual regulation of lin28a by Myc is necessary during zebrafish retina regeneration.J Cell Biol. 2019 Feb 4;218(2):489-507. doi: 10.1083/jcb.201802113. Epub 2019 Jan 3. J Cell Biol. 2019. PMID: 30606747 Free PMC article.
-
Suppression of a MEF2-KLF6 survival pathway by PKA signaling promotes apoptosis in embryonic hippocampal neurons.J Neurosci. 2012 Feb 22;32(8):2790-803. doi: 10.1523/JNEUROSCI.3609-11.2012. J Neurosci. 2012. PMID: 22357862 Free PMC article.
-
Retinal Ganglion Cell Survival and Axon Regeneration after Optic Nerve Injury: Role of Inflammation and Other Factors.Int J Mol Sci. 2022 Sep 5;23(17):10179. doi: 10.3390/ijms231710179. Int J Mol Sci. 2022. PMID: 36077577 Free PMC article. Review.
-
Stochastic cell-cycle entry and cell-state-dependent fate outputs of injury-reactivated tectal radial glia in zebrafish.Elife. 2019 Aug 23;8:e48660. doi: 10.7554/eLife.48660. Elife. 2019. PMID: 31442201 Free PMC article.
References
-
- Anderson PN, Campbell G, Zhang Y, Lieberman AR. Cellular and molecular correlates of the regeneration of adult mammalian CNS axons into peripheral nerve grafts. Prog Brain Res. 1998;117:211–232. - PubMed
-
- Barthel LK, Raymond PA. In situ hybridization studies of retinal neurons. Methods Enzymol. 2000;316:579–590. - PubMed
-
- Basi GS, Jacobson RD, Virag I, Schilling J, Skene JH. Primary structure and transcriptional regulation of GAP-43, a protein associated with nerve growth. Cell. 1987;49:785–791. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

