Antigenic changes in human albumin caused by reactivity with the occupational allergen diphenylmethane diisocyanate
- PMID: 20123080
- PMCID: PMC3106273
- DOI: 10.1016/j.ab.2010.01.037
Antigenic changes in human albumin caused by reactivity with the occupational allergen diphenylmethane diisocyanate
Abstract
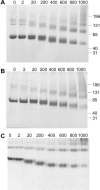
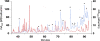
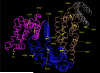
Diphenylmethane diisocyanate (MDI), the chemical commonly used as a cross-linking agent in commercial polyurethane production, is a well-recognized cause of asthma. Reaction products between MDI and "self" proteins are hypothesized to act as antigens capable of inducing airway inflammation and asthma; however, such MDI antigens remain incompletely understood. We used a variety of analytical methods to characterize the range of MDI-albumin reaction products that form under physiological conditions. Sites of MDI conjugation on antigenic MDI-albumin products, as defined by serum immunoglobulin G (IgG) from MDI-exposed workers, were determined by high-performance liquid chromatography (HPLC) followed by tandem mass spectrometry (MS/MS). The data identified 14 MDI conjugation sites (12 lysines and 2 asparagines) on human albumin and highlight reaction specificity for the second lysine in dilysine (KK) motifs, and this may be a common characteristic of "immune-sensitizing" chemicals. Several of the MDI conjugation sites are not conserved in albumin from other species, and this may suggest species differences in epitope specificity for self protein (albumin)-isocyanate conjugates. The study also describes new applications of contemporary proteomic methodology for characterizing and standardizing MDI-albumin conjugates destined for use in clinical research.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures


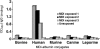
Similar articles
-
Determination of albumin adducts of 4,4'-methylenediphenyl diisocyanate after specific inhalative challenge tests in workers.Toxicol Lett. 2016 Oct 17;260:46-51. doi: 10.1016/j.toxlet.2016.08.008. Epub 2016 Aug 10. Toxicol Lett. 2016. PMID: 27521498
-
New isocyanate-specific albumin adducts of 4,4'-methylenediphenyl diisocyanate (MDI) in rats.Chem Res Toxicol. 2009 Dec;22(12):1975-83. doi: 10.1021/tx900270z. Chem Res Toxicol. 2009. PMID: 19928878
-
Indirect assessment of 4,4'-diphenylmethane diisocyanate (MDI) exposure by evaluation of specific humoral immune responses to MDI conjugated to human serum albumin.Am J Ind Med. 1998 May;33(5):471-7. doi: 10.1002/(sici)1097-0274(199805)33:5<471::aid-ajim6>3.0.co;2-v. Am J Ind Med. 1998. PMID: 9557170
-
Carcinogenic risk of toluene diisocyanate and 4,4'-methylenediphenyl diisocyanate: epidemiological and experimental evidence.Crit Rev Toxicol. 2001 Nov;31(6):737-72. doi: 10.1080/20014091111974. Crit Rev Toxicol. 2001. PMID: 11763481 Review.
-
Developments in laboratory diagnostics for isocyanate asthma.Curr Opin Allergy Clin Immunol. 2007 Apr;7(2):138-45. doi: 10.1097/ACI.0b013e3280895d22. Curr Opin Allergy Clin Immunol. 2007. PMID: 17351466 Free PMC article. Review.
Cited by
-
Molecular determinants of humoral immune specificity for the occupational allergen, methylene diphenyl diisocyanate.Mol Immunol. 2013 Jun;54(2):233-7. doi: 10.1016/j.molimm.2012.11.017. Epub 2013 Jan 4. Mol Immunol. 2013. PMID: 23295252 Free PMC article.
-
IgE allergy diagnostics and other relevant tests in allergy, a World Allergy Organization position paper.World Allergy Organ J. 2020 Feb 25;13(2):100080. doi: 10.1016/j.waojou.2019.100080. eCollection 2020 Feb. World Allergy Organ J. 2020. PMID: 32128023 Free PMC article.
-
Environmental isocyanate-induced asthma: morphologic and pathogenetic aspects of an increasing occupational disease.Int J Environ Res Public Health. 2011 Sep;8(9):3672-87. doi: 10.3390/ijerph8093672. Epub 2011 Sep 9. Int J Environ Res Public Health. 2011. PMID: 22016709 Free PMC article. Review.
-
Molecular Characterization and Experimental Utility of Monoclonal Antibodies with Specificity for Aliphatic Di- and Polyisocyanates.Monoclon Antib Immunodiagn Immunother. 2020 Jun;39(3):66-73. doi: 10.1089/mab.2020.0006. Epub 2020 Apr 17. Monoclon Antib Immunodiagn Immunother. 2020. PMID: 32302507 Free PMC article.
-
Toluene diisocyanate reactivity with glutathione across a vapor/liquid interface and subsequent transcarbamoylation of human albumin.Chem Res Toxicol. 2011 Oct 17;24(10):1686-93. doi: 10.1021/tx2002433. Epub 2011 Aug 10. Chem Res Toxicol. 2011. PMID: 21806041 Free PMC article.
References
-
- Wisnewski A, Redlich C, Mapp C, Bernstein D. Polyisocyanates. Informa Healthcare; London: 2006.
-
- Redlich CA, Bello D, Wisnewski AV. Health effects of isocyanates. In: Rom WN, editor. Environmental and Occupational Medicine. Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 502–515.
-
- Redlich CA, Bello D, Woskie SR, Streicher RP. Measurements of airborne methylene diphenyl diisocyanate concentration in the US workplace. J. Occup. Environ. Hyg. 2009;6:D82–D83. comment. Author reply, D83–D85. - PubMed
-
- Robert A, Ducos P, Francin JM, Marsan P. Biological monitoring of workers exposed to 4,4′-methylenediphenyl diisocyanate (MDI) in 19 French polyurethane industries. Intl. Arch. Occup. Environ. Health. 2007;80:412–422. - PubMed
-
- Sabbioni G, Wesp H, Lewalter J, Rumler R. Determination of isocyanate biomarkers in construction site workers. Biomarkers. 2007;12:468–483. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

