Cytokine induced expression of programmed death ligands in human neutrophils
- PMID: 20123111
- PMCID: PMC2849642
- DOI: 10.1016/j.imlet.2010.01.006
Cytokine induced expression of programmed death ligands in human neutrophils
Abstract
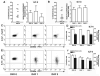
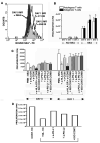
Recent evidence indicates that human neutrophils can serve as non-professional antigen presenting cells (APC). Although expression of MHC class II and co-stimulatory molecules on human neutrophils is limited, these molecules can be significantly induced following in vitro exposure to the cytokines IFNgamma and GM-CSF. Since professional APCs such as dendritic cells express both co-stimulatory and co-inhibitory molecules for activation and regulation of adaptive immunity, we determined whether cytokines induce increased expression of specific co-signaling molecules on human neutrophils. We report here that circulating human neutrophils express co-inhibitory molecules such as immunoglobulin-like transcript (ILT) 4 and 5, and also comparatively low and highly variable levels of ILT2 and ILT3, but the expression of these ILTs was not significantly changed by cytokine treatment. In contrast, we demonstrate for the first time that human peripheral blood neutrophils, although do not express the co-inhibitory molecule, programmed death ligand (PD-L) 1 on their surface, can express this molecule at moderate levels following cytokine exposure. Although moderate PD-L1 levels on healthy volunteers' neutrophils were not inhibitory to T cells, our findings do not exclude a possible robust increase in neutrophil PD-L1 expression in pathological conditions with immunosuppressive functions. These results suggest a possible immunoregulatory role for human neutrophils in adaptive immunity.
Copyright 2010 Elsevier B.V. All rights reserved.
Figures


References
-
- Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. 2007;219:88–102. - PubMed
-
- Steinman RM. Dendritic cells: understanding immunogenicity. Eur J Immunol. 2007;37 (Suppl 1):S53–60. - PubMed
-
- Adler HS, Steinbrink K. Tolerogenic dendritic cells in health and disease: friend and foe! Eur J Dermatol. 2007;17:476–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

