Lipidomic study of intracellular Singapore grouper iridovirus
- PMID: 20123143
- PMCID: PMC7126382
- DOI: 10.1016/j.virol.2010.01.016
Lipidomic study of intracellular Singapore grouper iridovirus
Abstract
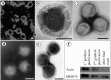
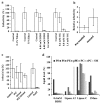
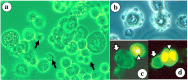
Singapore grouper iridoviruses (SGIV) infected grouper cells release few enveloped extracellular viruses by budding and many unenveloped intracellular viruses following cell lysis. The lipid composition and function of such unenveloped intracellular viruses remain unknown. Detergent treatment of the intracellular viruses triggered the loss of viral lipids, capsid proteins and infectivity. Enzymatic digestion of the viral lipids with phospholipases and sphingomyelinase retained the viral capsid proteins but reduced infectivity. Over 220 lipid species were identified and quantified from the viruses and its producer cells by electrospray ionization mass spectrometry. Ten caspid proteins that dissociated from the viruses following the detergent treatments were identified by MALDI-TOF/TOF-MS/MS. Five of them were demonstrated to be lipid-binding proteins. This is the first research detailing the lipidome and lipid-protein interactions of an unenveloped virus. The identified lipid species and lipid-binding proteins will facilitate further studies of the viral assembly, egress and entry.
Copyright 2010 Elsevier Inc. All rights reserved.
Figures



Similar articles
-
Singapore Grouper Iridovirus Disturbed Glycerophospholipids Homeostasis: Cytosolic Phospholipase A2 Was Essential for Virus Replication.Int J Mol Sci. 2021 Nov 22;22(22):12597. doi: 10.3390/ijms222212597. Int J Mol Sci. 2021. PMID: 34830477 Free PMC article.
-
iTRAQ analysis of Singapore grouper iridovirus infection in a grouper embryonic cell line.J Gen Virol. 2008 Nov;89(Pt 11):2869-2876. doi: 10.1099/vir.0.2008/003681-0. J Gen Virol. 2008. PMID: 18931085
-
In situ hybridization of a marine fish virus, Singapore grouper iridovirus with a nucleic acid probe of major capsid protein.J Virol Methods. 2004 May;117(2):123-8. doi: 10.1016/j.jviromet.2004.01.002. J Virol Methods. 2004. PMID: 15041208
-
Matrix-assisted laser desorption and ionization time-of-flight (MALDI-TOF) mass spectrometry in lipid and phospholipid research.Prog Lipid Res. 2004 Sep;43(5):449-88. doi: 10.1016/j.plipres.2004.08.001. Prog Lipid Res. 2004. PMID: 15458815 Review.
-
Surface analysis of lipids by mass spectrometry: more than just imaging.Prog Lipid Res. 2013 Oct;52(4):329-53. doi: 10.1016/j.plipres.2013.04.005. Epub 2013 Apr 24. Prog Lipid Res. 2013. PMID: 23623802 Review.
Cited by
-
Diet and Life Stage-Associated Lipidome Remodeling in Atlantic Salmon.J Agric Food Chem. 2021 Mar 31;69(12):3787-3796. doi: 10.1021/acs.jafc.0c07281. Epub 2021 Mar 23. J Agric Food Chem. 2021. PMID: 33754702 Free PMC article.
-
Singapore Grouper Iridovirus Disturbed Glycerophospholipids Homeostasis: Cytosolic Phospholipase A2 Was Essential for Virus Replication.Int J Mol Sci. 2021 Nov 22;22(22):12597. doi: 10.3390/ijms222212597. Int J Mol Sci. 2021. PMID: 34830477 Free PMC article.
-
Fluorescence, Circular Dichroism and Mass Spectrometry as Tools to Study Virus Structure.Subcell Biochem. 2024;105:207-245. doi: 10.1007/978-3-031-65187-8_6. Subcell Biochem. 2024. PMID: 39738948 Review.
-
Segmentation and Comparative Modeling in an 8.6-Å Cryo-EM Map of the Singapore Grouper Iridovirus.Structure. 2019 Oct 1;27(10):1561-1569.e4. doi: 10.1016/j.str.2019.08.002. Epub 2019 Aug 22. Structure. 2019. PMID: 31447288 Free PMC article.
-
Singapore Grouper Iridovirus ORF75R is a Scaffold Protein Essential for Viral Assembly.Sci Rep. 2015 Aug 19;5:13151. doi: 10.1038/srep13151. Sci Rep. 2015. PMID: 26286371 Free PMC article.
References
-
- Balange-Orange N., Devauchelle G. Lipid composition of an iridescent virus type 6 (CIV) Arch. Virol. 1982;73:363–367. - PubMed
-
- Barrero-Villar M., Barroso-González J., Cabrero J.R., Gordón-Alonso M., Alvarez-Losada S., Muñoz-Fernández M.A., Sánchez-Madrid F., Valenzuela-Fernández A. PI4P5-kinase Ialpha is required for efficient HIV-1 entry and infection of T cells. J. Immunol. 2008;181:6882–6888. - PubMed
-
- Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. - PubMed
-
- Braunwald J., Nonnenmacher H., Tripier-Darcy F. Ultrastructural and biochemical study of frog virus 3 uptake by BHK-21 cells. J. Gen. Virol. 1985;66:283–293. - PubMed
-
- Bravo I.G., Crusius K., Alonso A. The E5 protein of the human papillomavirus type 16 modulates composition and dynamics of membrane lipids in keratinocytes. Arch. Virol. 2005;150:231–246. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

