Computational model of steroidogenesis in human H295R cells to predict biochemical response to endocrine-active chemicals: model development for metyrapone
- PMID: 20123619
- PMCID: PMC2831928
- DOI: 10.1289/ehp.0901107
Computational model of steroidogenesis in human H295R cells to predict biochemical response to endocrine-active chemicals: model development for metyrapone
Erratum in
- Environ Health Perspect. 2011 Jan;119(1):A11
Abstract
Background: An in vitro steroidogenesis assay using the human adrenocortical carcinoma cell line H295R is being evaluated as a possible screening assay to detect and assess the impact of endocrine-active chemicals (EACs) capable of altering steroid biosynthesis. Data interpretation and their quantitative use in human and ecological risk assessments can be enhanced with mechanistic computational models to help define mechanisms of action and improve understanding of intracellular concentration-response behavior.
Objectives: The goal of this study was to develop a mechanistic computational model of the metabolic network of adrenal steroidogenesis to estimate the synthesis and secretion of adrenal steroids in human H295R cells and their biochemical response to steroidogenesis-disrupting EAC.
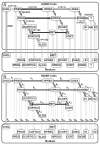
Methods: We developed a deterministic model that describes the biosynthetic pathways for the conversion of cholesterol to adrenal steroids and the kinetics for enzyme inhibition by metryrapone (MET), a model EAC. Using a nonlinear parameter estimation method, the model was fitted to the measurements from an in vitro steroidogenesis assay using H295R cells.
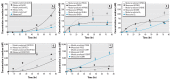
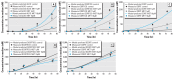
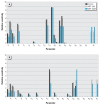
Results: Model-predicted steroid concentrations in cells and culture medium corresponded well to the time-course measurements from control and MET-exposed cells. A sensitivity analysis indicated the parameter uncertainties and identified transport and metabolic processes that most influenced the concentrations of primary adrenal steroids, aldosterone and cortisol.
Conclusions: Our study demonstrates the feasibility of using a computational model of steroidogenesis to estimate steroid concentrations in vitro. This capability could be useful to help define mechanisms of action for poorly characterized chemicals and mixtures in support of predictive hazard and risk assessments with EACs.
Conflict of interest statement
N.T. and M.Y. are employed by Mitsubishi Tanabe Pharma Corporation. The other authors declare they have no competing financial interests.
Figures
Similar articles
-
Mechanistic computational model of steroidogenesis in H295R cells: role of oxysterols and cell proliferation to improve predictability of biochemical response to endocrine active chemical--metyrapone.Toxicol Sci. 2011 Sep;123(1):80-93. doi: 10.1093/toxsci/kfr167. Epub 2011 Jul 1. Toxicol Sci. 2011. PMID: 21725065
-
Steroid profiling in H295R cells to identify chemicals potentially disrupting the production of adrenal steroids.Toxicology. 2017 Apr 15;381:51-63. doi: 10.1016/j.tox.2017.02.010. Epub 2017 Feb 22. Toxicology. 2017. PMID: 28235592
-
LC-MS/MS based profiling and dynamic modelling of the steroidogenesis pathway in adrenocarcinoma H295R cells.Toxicol In Vitro. 2018 Oct;52:332-341. doi: 10.1016/j.tiv.2018.07.002. Epub 2018 Jul 11. Toxicol In Vitro. 2018. PMID: 30017865
-
Human adrenocortical carcinoma cell line (NCI-H295R): An in vitro screening model for the assessment of endocrine disruptors' actions on steroidogenesis with an emphasis on cell ultrastructural features.Acta Histochem. 2022 Jul;124(5):151912. doi: 10.1016/j.acthis.2022.151912. Epub 2022 Jun 1. Acta Histochem. 2022. PMID: 35661985 Review.
-
New mechanisms of adrenostatic compounds in a human adrenocortical cancer cell line.Eur J Clin Invest. 2000 Dec;30 Suppl 3:76-82. doi: 10.1046/j.1365-2362.2000.0300s3076.x. Eur J Clin Invest. 2000. PMID: 11281374 Review.
Cited by
-
A computational model to predict rat ovarian steroid secretion from in vitro experiments with endocrine disruptors.PLoS One. 2013;8(1):e53891. doi: 10.1371/journal.pone.0053891. Epub 2013 Jan 11. PLoS One. 2013. PMID: 23326527 Free PMC article.
-
Development of a prioritization method for chemical-mediated effects on steroidogenesis using an integrated statistical analysis of high-throughput H295R data.Regul Toxicol Pharmacol. 2019 Dec;109:104510. doi: 10.1016/j.yrtph.2019.104510. Epub 2019 Oct 29. Regul Toxicol Pharmacol. 2019. PMID: 31676319 Free PMC article.
-
Evaluating structure-based activity in a high-throughput assay for steroid biosynthesis.Comput Toxicol. 2022 Nov 1;24:1-23. doi: 10.1016/j.comtox.2022.100245. Comput Toxicol. 2022. PMID: 37841081 Free PMC article.
-
Estimation of the Mechanism of Adrenal Action of Endocrine-Disrupting Compounds Using a Computational Model of Adrenal Steroidogenesis in NCI-H295R Cells.J Toxicol. 2016;2016:4041827. doi: 10.1155/2016/4041827. Epub 2016 Feb 17. J Toxicol. 2016. PMID: 27057163 Free PMC article.
-
Metyrapone, an inhibitor of cytochrome oxidases, does not affect viability in a neuroblastoma cell model of bilirubin toxicity.Mol Genet Metab Rep. 2014 Apr 25;1:197-202. doi: 10.1016/j.ymgmr.2014.04.002. eCollection 2014. Mol Genet Metab Rep. 2014. PMID: 27896088 Free PMC article.
References
-
- Agarwal AK, Auchus RJ. Minireview: cellular redox state regulates hydroxysteroid dehydrogenase activity and intracellular hormone potency. Endocrinology. 2005;146:2531–2538. - PubMed
-
- Becker S, Chubb C, Ewing L. Mathematical model of steroidogenesis in rat and rabbit testes. Am J Physiol. 1980;239:R184–R195. - PubMed
-
- Breen MS, Villeneuve DL, Breen M, Ankley GT, Conolly RB. Mechanistic computational model of ovarian steroidogenesis to predict biochemical responses to endocrine active compounds. Ann Biomed Eng. 2007;35:970–981. - PubMed
-
- Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. - PubMed
-
- Chang TY, Chang CC, Ohgami N, Yamauchi Y. Cholesterol sensing, trafficking, and esterification. Annu Rev Cell Dev Biol. 2006;22:129–157. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

