Vibrio cholerae phosphoenolpyruvate phosphotransferase system control of carbohydrate transport, biofilm formation, and colonization of the germfree mouse intestine
- PMID: 20123708
- PMCID: PMC2849402
- DOI: 10.1128/IAI.01356-09
Vibrio cholerae phosphoenolpyruvate phosphotransferase system control of carbohydrate transport, biofilm formation, and colonization of the germfree mouse intestine
Abstract
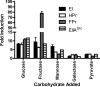
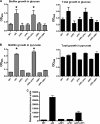
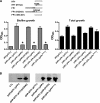
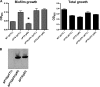
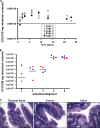
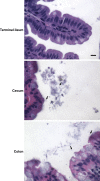
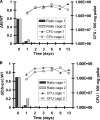
The bacterial phosphoenolpyruvate phosphotransferase system (PTS) is a highly conserved phosphotransfer cascade whose components modulate many cellular functions in response to carbohydrate availability. Here, we further elucidate PTS control of Vibrio cholerae carbohydrate transport and activation of biofilm formation on abiotic surfaces. We then define the role of the PTS in V. cholerae colonization of the adult germfree mouse intestine. We report that V. cholerae colonizes both the small and large intestines of the mouse in a distribution that does not change over the course of a month-long experiment. Because V. cholerae possesses many PTS-independent carbohydrate transporters, the PTS is not essential for bacterial growth in vitro. However, we find that the PTS is essential for colonization of the germfree adult mouse intestine and that this requirement is independent of PTS regulation of biofilm formation. Therefore, competition for PTS substrates may be a dominant force in the success of V. cholerae as an intestinal pathogen. Because the PTS plays a role in colonization of environmental surfaces and the mammalian intestine, we propose that it may be essential to successful transit of V. cholerae through its life cycle of pathogenesis and environmental persistence.
Figures

Similar articles
-
Removal of a Membrane Anchor Reveals the Opposing Regulatory Functions of Vibrio cholerae Glucose-Specific Enzyme IIA in Biofilms and the Mammalian Intestine.mBio. 2018 Sep 4;9(5):e00858-18. doi: 10.1128/mBio.00858-18. mBio. 2018. PMID: 30181246 Free PMC article.
-
The phosphoenolpyruvate phosphotransferase system regulates Vibrio cholerae biofilm formation through multiple independent pathways.J Bacteriol. 2010 Jun;192(12):3055-67. doi: 10.1128/JB.00213-10. Epub 2010 Apr 16. J Bacteriol. 2010. PMID: 20400550 Free PMC article.
-
Glucose-specific enzyme IIA has unique binding partners in the vibrio cholerae biofilm.mBio. 2012 Nov 6;3(6):e00228-12. doi: 10.1128/mBio.00228-12. mBio. 2012. PMID: 23131828 Free PMC article.
-
Sophisticated Regulation of Transcriptional Factors by the Bacterial Phosphoenolpyruvate: Sugar Phosphotransferase System.J Mol Biol. 2017 Mar 24;429(6):773-789. doi: 10.1016/j.jmb.2017.02.006. Epub 2017 Feb 13. J Mol Biol. 2017. PMID: 28202392 Review.
-
Vibrio cholerae interactions with the gastrointestinal tract: lessons from animal studies.Curr Top Microbiol Immunol. 2009;337:37-59. doi: 10.1007/978-3-642-01846-6_2. Curr Top Microbiol Immunol. 2009. PMID: 19812979 Review.
Cited by
-
NagC represses N-acetyl-glucosamine utilization genes in Vibrio fischeri within the light organ of Euprymna scolopes.Front Microbiol. 2015 Jul 17;6:741. doi: 10.3389/fmicb.2015.00741. eCollection 2015. Front Microbiol. 2015. PMID: 26236308 Free PMC article.
-
Vibrio cholerae genomic diversity within and between patients.Microb Genom. 2017 Dec;3(12):e000142. doi: 10.1099/mgen.0.000142. Microb Genom. 2017. PMID: 29306353 Free PMC article.
-
The role of the universal sugar transport system components PtsI (EI) and PtsH (HPr) in Enterococcus faecium.FEMS Microbes. 2024 Jun 3;5:xtae018. doi: 10.1093/femsmc/xtae018. eCollection 2024. FEMS Microbes. 2024. PMID: 38988831 Free PMC article.
-
Advance typing of Vibrio parahaemolyticus through the mtlA and aer gene: A high-resolution, cost-effective approach.Heliyon. 2024 Feb 5;10(3):e25642. doi: 10.1016/j.heliyon.2024.e25642. eCollection 2024 Feb 15. Heliyon. 2024. PMID: 38356529 Free PMC article.
-
A mannose-sensing AraC-type transcriptional activator regulates cell-cell aggregation of Vibrio cholerae.NPJ Biofilms Microbiomes. 2022 Aug 20;8(1):65. doi: 10.1038/s41522-022-00331-x. NPJ Biofilms Microbiomes. 2022. PMID: 35987769 Free PMC article.
References
-
- Berg, T., S. Schild, and J. Reidl. 2007. Regulation of the chitobiose-phosphotransferase system in Vibrio cholerae. Arch. Microbiol. 187:433-439. - PubMed
-
- Brown, J. H., and L. Brenn. 1931. A method for the differential staining of Gram-positive and Gram-negative bacteria in tissue sections. Bull. Johns Hopkins Hosp. 48:69-73.
-
- Chiang, S. L., and J. J. Mekalanos. 1998. Use of signature-tagged transposon mutagenesis to identify Vibrio cholerae genes critical for colonization. Mol. Microbiol. 27:797-805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

