Critical role of dispensable genes in Mycoplasma agalactiae interaction with mammalian cells
- PMID: 20123713
- PMCID: PMC2849427
- DOI: 10.1128/IAI.01195-09
Critical role of dispensable genes in Mycoplasma agalactiae interaction with mammalian cells
Abstract
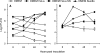
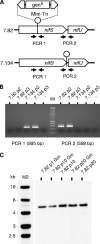
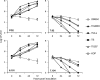
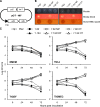
Mycoplasmas are minimal bacteria whose genomes barely exceed the smallest amount of information required to sustain autonomous life. Despite this apparent simplicity, several mycoplasmas are successful pathogens of humans and animals, in which they establish intimate interactions with epithelial cells at mucosal surfaces. To identify biological functions mediating mycoplasma interactions with mammalian cells, we produced a library of transposon knockout mutants in the ruminant pathogen Mycoplasma agalactiae and used this library to identify mutants displaying a growth-deficient pheonotype in cell culture. M. agalactiae mutants displaying a 3-fold reduction in CFU titers to nearly complete extinction in coculture with HeLa cells were identified. Mapping of transposon insertion sites revealed 18 genomic regions putatively involved in the interaction of M. agalactiae with HeLa cells. Several of these regions encode proteins with features of membrane lipoproteins and/or were involved in horizontal gene transfer with phylogenetically distant pathogenic mycoplasmas of ruminants. Two mutants with the most extreme phenotype carry a transposon in a genomic region designated the NIF locus which encodes homologues of SufS and SufU, two proteins presumably involved in [Fe-S] cluster biosynthesis in Gram-positive bacteria. Complementation studies confirmed the conditional essentiality of the NIF locus, which was found to be critical for proliferation in the presence of HeLa cells and several other mammalian cell lines but dispensable for axenic growth. While our results raised questions regarding essential functions in mycoplasmas, they also provide a means for studying the role of mycoplasmas as minimal pathogens.
Figures

References
-
- Bergonier, D., X. Berthelot, and F. Poumarat. 1997. Contagious agalactia of small ruminants: current knowledge concerning epidemiology, diagnosis and control. Rev. Sci. Tech. 16:848-873. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

