Pathogenicity islands PAPI-1 and PAPI-2 contribute individually and synergistically to the virulence of Pseudomonas aeruginosa strain PA14
- PMID: 20123716
- PMCID: PMC2849418
- DOI: 10.1128/IAI.00621-09
Pathogenicity islands PAPI-1 and PAPI-2 contribute individually and synergistically to the virulence of Pseudomonas aeruginosa strain PA14
Abstract
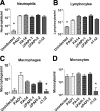
Pseudomonas aeruginosa is a leading cause of hospital-acquired pneumonia and severe chronic lung infections in cystic fibrosis patients. The reference strains PA14 and PAO1 have been studied extensively, revealing that PA14 is more virulent than PAO1 in diverse infection models. Among other factors, this may be due to two pathogenicity islands, PAPI-1 and PAPI-2, both present in PA14 but not in PAO1. We compared the global contributions to virulence of PAPI-1 and PAPI-2, rather than that of individual island-borne genes, using murine models of acute pneumonia and bacteremia. Three isogenic island-minus mutants (PAPI-1-minus, PAPI-2-minus, and PAPI-1-minus, PAPI-2-minus mutants) were compared with the wild-type parent strain PA14 and with PAO1. Our results showed that both islands contributed significantly to the virulence of PA14 in acute pneumonia and bacteremia models. However, in contrast to the results for the bacteremia model, where each island was found to contribute individually, loss of the 108-kb PAPI-1 island alone was insufficient to measurably attenuate the mutant in the acute pneumonia model. Nevertheless, the double mutant was substantially more attenuated, and exhibited a lesser degree of virulence, than even PAO1 in the acute pneumonia model. In particular, its ability to disseminate from the lungs to the bloodstream was markedly inhibited. We conclude that both PAPI-1 and PAPI-2 contribute directly and synergistically in a major way to the virulence of PA14, and we suggest that analysis of island-minus strains may be a more appropriate way than individual gene knockouts to assess the contributions to virulence of large, horizontally acquired segments of DNA.
Figures
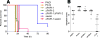



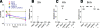
Similar articles
-
The broad host range pathogen Pseudomonas aeruginosa strain PA14 carries two pathogenicity islands harboring plant and animal virulence genes.Proc Natl Acad Sci U S A. 2004 Feb 24;101(8):2530-5. doi: 10.1073/pnas.0304622101. Proc Natl Acad Sci U S A. 2004. PMID: 14983043 Free PMC article.
-
Conjugative type IVb pilus recognizes lipopolysaccharide of recipient cells to initiate PAPI-1 pathogenicity island transfer in Pseudomonas aeruginosa.BMC Microbiol. 2017 Feb 7;17(1):31. doi: 10.1186/s12866-017-0943-4. BMC Microbiol. 2017. PMID: 28173753 Free PMC article.
-
Hybrid pathogenicity island PAGI-5 contributes to the highly virulent phenotype of a Pseudomonas aeruginosa isolate in mammals.J Bacteriol. 2008 Nov;190(21):7130-40. doi: 10.1128/JB.00785-08. Epub 2008 Aug 29. J Bacteriol. 2008. PMID: 18757543 Free PMC article.
-
Pseudomonas aeruginosa: breaking down barriers.Curr Genet. 2016 Feb;62(1):109-13. doi: 10.1007/s00294-015-0522-x. Epub 2015 Sep 25. Curr Genet. 2016. PMID: 26407972 Free PMC article. Review.
-
Pseudomonas aeruginosa reference strains PAO1 and PA14: A genomic, phenotypic, and therapeutic review.Front Microbiol. 2022 Oct 13;13:1023523. doi: 10.3389/fmicb.2022.1023523. eCollection 2022. Front Microbiol. 2022. PMID: 36312971 Free PMC article. Review.
Cited by
-
From genotype to phenotype: adaptations of Pseudomonas aeruginosa to the cystic fibrosis environment.Microb Genom. 2021 Mar;7(3):mgen000513. doi: 10.1099/mgen.0.000513. Epub 2021 Feb 2. Microb Genom. 2021. PMID: 33529147 Free PMC article. Review.
-
Pseudomonas aeruginosa Pangenome: Core and Accessory Genes of a Highly Resourceful Opportunistic Pathogen.Adv Exp Med Biol. 2022;1386:3-28. doi: 10.1007/978-3-031-08491-1_1. Adv Exp Med Biol. 2022. PMID: 36258067
-
Pseudomonas aeruginosa AES-1 exhibits increased virulence gene expression during chronic infection of cystic fibrosis lung.PLoS One. 2011;6(9):e24526. doi: 10.1371/journal.pone.0024526. Epub 2011 Sep 15. PLoS One. 2011. PMID: 21935417 Free PMC article.
-
Genome-scale model of Pseudomonas aeruginosa metabolism unveils virulence and drug potentiation.Commun Biol. 2023 Feb 10;6(1):165. doi: 10.1038/s42003-023-04540-8. Commun Biol. 2023. PMID: 36765199 Free PMC article.
-
Carbapenemases on the move: it's good to be on ICEs.Mob DNA. 2018 Dec 19;9:37. doi: 10.1186/s13100-018-0141-4. eCollection 2018. Mob DNA. 2018. PMID: 30574213 Free PMC article.
References
-
- Antonopoulou, A., M. Raftogiannis, E. J. Giamarellos-Bourboulis, P. Koutoukas, L. Sabracos, M. Mouktaroudi, T. Adamis, I. Tzepi, H. Giamarellou, and E. E. Douzinas. 2007. Early apoptosis of blood monocytes is a determinant of survival in experimental sepsis by multi-drug-resistant Pseudomonas aeruginosa. Clin. Exp. Immunol. 149:103-108. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

