Virulence plasmid harbored by uropathogenic Escherichia coli functions in acute stages of pathogenesis
- PMID: 20123719
- PMCID: PMC2849428
- DOI: 10.1128/IAI.01260-09
Virulence plasmid harbored by uropathogenic Escherichia coli functions in acute stages of pathogenesis
Abstract
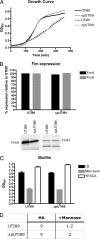
Urinary tract infections (UTIs), the majority of which are caused by uropathogenic Escherichia coli (UPEC), afflict nearly 60% of women within their lifetimes. Studies in mice and humans have revealed that UPEC strains undergo a complex pathogenesis cycle that involves both the formation of intracellular bacterial communities (IBC) and the colonization of extracellular niches. Despite the commonality of the UPEC pathogenesis cycle, no specific urovirulence genetic profile has been determined; this is likely due to the fluid nature of the UPEC genome as the result of horizontal gene transfer and numerous genes of unknown function. UTI89 has a large extrachromosomal element termed pUTI89 with many characteristics of UPEC pathogenicity islands and that likely arose due to horizontal gene transfer. The pUTI89 plasmid has characteristics of both F plasmids and other known virulence plasmids. We sought to determine whether pUTI89 is important for virulence. Both in vitro and in vivo assays were used to examine the function of pUTI89 using plasmid-cured UTI89. No differences were observed between UTI89 and plasmid-cured UTI89 based on growth, type 1 pilus expression, or biofilm formation. However, in a mouse model of UTI, a significant decrease in bacterial invasion, CFU and IBC formation of the pUTI89-cured strain was observed at early time points postinfection compared to the wild type. Through directed deletions of specific operons on pUTI89, the cjr operon was partially implicated in this observed defect. Our findings implicate pUTI89 in the early aspects of infection.
Figures

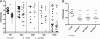
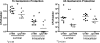

Similar articles
-
Metabolic Requirements of Escherichia coli in Intracellular Bacterial Communities during Urinary Tract Infection Pathogenesis.mBio. 2016 Apr 12;7(2):e00104-16. doi: 10.1128/mBio.00104-16. mBio. 2016. PMID: 27073089 Free PMC article.
-
Comparison of phenotypic and genetic traits of ESBL-producing UPEC strains causing recurrent or single episode UTI in postmenopausal women.Ann Clin Microbiol Antimicrob. 2025 Feb 7;24(1):11. doi: 10.1186/s12941-025-00779-7. Ann Clin Microbiol Antimicrob. 2025. PMID: 39920666 Free PMC article.
-
Discovery of New Genes Involved in Curli Production by a Uropathogenic Escherichia coli Strain from the Highly Virulent O45:K1:H7 Lineage.mBio. 2018 Aug 21;9(4):e01462-18. doi: 10.1128/mBio.01462-18. mBio. 2018. PMID: 30131362 Free PMC article.
-
Pathogenomics of uropathogenic Escherichia coli.Indian J Med Microbiol. 2012 Apr-Jun;30(2):141-9. doi: 10.4103/0255-0857.96657. Indian J Med Microbiol. 2012. PMID: 22664427 Review.
-
Virulence factors of uropathogenic E. coli and their interaction with the host.Adv Microb Physiol. 2014;65:337-72. doi: 10.1016/bs.ampbs.2014.08.006. Epub 2014 Nov 4. Adv Microb Physiol. 2014. PMID: 25476769 Review.
Cited by
-
Environmental and genetic determinants of plasmid mobility in pathogenic Escherichia coli.Sci Adv. 2020 Jan 24;6(4):eaax3173. doi: 10.1126/sciadv.aax3173. eCollection 2020 Jan. Sci Adv. 2020. PMID: 32042895 Free PMC article.
-
The Complete Sequence and Comparative Analysis of a Multidrug-Resistance and Virulence Multireplicon IncFII Plasmid pEC302/04 from an Extraintestinal Pathogenic Escherichia coli EC302/04 Indicate Extensive Diversity of IncFII Plasmids.Front Microbiol. 2016 Jan 11;6:1547. doi: 10.3389/fmicb.2015.01547. eCollection 2015. Front Microbiol. 2016. PMID: 26793180 Free PMC article.
-
Host-pathogen checkpoints and population bottlenecks in persistent and intracellular uropathogenic Escherichia coli bladder infection.FEMS Microbiol Rev. 2012 May;36(3):616-48. doi: 10.1111/j.1574-6976.2012.00339.x. FEMS Microbiol Rev. 2012. PMID: 22404313 Free PMC article. Review.
-
Genome Dynamics of Escherichia coli during Antibiotic Treatment: Transfer, Loss, and Persistence of Genetic Elements In situ of the Infant Gut.Front Cell Infect Microbiol. 2017 Apr 12;7:126. doi: 10.3389/fcimb.2017.00126. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28447026 Free PMC article.
-
Evolutionary genomic analyses of canine E. coli infections identify a relic capsular locus associated with resistance to multiple classes of antimicrobials.Appl Environ Microbiol. 2024 Aug 21;90(8):e0035424. doi: 10.1128/aem.00354-24. Epub 2024 Jul 16. Appl Environ Microbiol. 2024. PMID: 39012166 Free PMC article.
References
-
- Anderson, G. G., J. J. Palermo, J. D. Schilling, R. Roth, J. Heuser, and S. J. Hultgren. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105-107. - PubMed
-
- Andrews, S. C., A. K. Robinson, and F. Rodriguez-Quinones. 2003. Bacterial iron homeostasis. FEMS Microbiol. Rev. 27:215-237. - PubMed
-
- Bai, G., E. Smith, A. Golubov, J. Pata, and K. A. McDonough. 2007. Differential gene regulation in Yersinia pestis versus Yersinia pseudotuberculosis: effects of hypoxia and potential role of a plasmid regulator. Adv. Exp. Med. Biol. 603:131-144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

