Reactive microgliosis: extracellular micro-calpain and microglia-mediated dopaminergic neurotoxicity
- PMID: 20123724
- PMCID: PMC2860703
- DOI: 10.1093/brain/awp333
Reactive microgliosis: extracellular micro-calpain and microglia-mediated dopaminergic neurotoxicity
Abstract
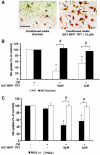
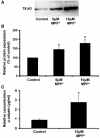
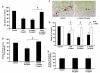
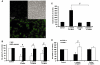
Microglia, the innate immune cells in the brain, can become chronically activated in response to dopaminergic neuron death, fuelling a self-renewing cycle of microglial activation followed by further neuron damage (reactive microgliosis), which is implicated in the progressive nature of Parkinson's disease. Here, we use an in vitro approach to separate neuron injury factors from the cellular actors of reactive microgliosis and discover molecular signals responsible for chronic and toxic microglial activation. Upon injury with the dopaminergic neurotoxin 1-methyl-4-phenylpyridinium, N27 cells (dopaminergic neuron cell line) released soluble neuron injury factors that activated microglia and were selectively toxic to dopaminergic neurons in mixed mesencephalic neuron-glia cultures through nicotinamide adenine dinucleotide phosphate oxidase. mu-Calpain was identified as a key signal released from damaged neurons, causing selective dopaminergic neuron death through activation of microglial nicotinamide adenine dinucleotide phosphate oxidase and superoxide production. These findings suggest that dopaminergic neurons may be inherently susceptible to the pro-inflammatory effects of neuron damage, i.e. reactive microgliosis, providing much needed insight into the chronic nature of Parkinson's disease.
Figures


References
-
- Block ML, Hong JS. Microglia and inflammation-mediated neurodegeneration: multiple triggers with a common mechanism. Prog Neurobiol. 2005;76:77–8. - PubMed
-
- Block ML, Hong JS. Chronic microglial activation and progressive dopaminergic neurotoxicity. Biochem Soc Trans. 2007;35:1127–32. - PubMed
-
- Block ML, Li G, Qin L, Wu X, Pei Z, Wang T, et al. Potent regulation of microglia-derived oxidative stress and dopaminergic neuron survival: substance P vs. dynorphin. Faseb J. 2006;20:251–8. - PubMed
-
- Block ML, Wu X, Pei Z, Li G, Wang T, Qin L, et al. Nanometer size diesel exhaust particles are selectively toxic to dopaminergic neurons: the role of microglia, phagocytosis, and NADPH oxidase. Faseb J. 2004;18:1618–20. - PubMed
-
- Block ML, Zecca L, Hong JS. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

