TRAP150 activates pre-mRNA splicing and promotes nuclear mRNA degradation
- PMID: 20123736
- PMCID: PMC2879504
- DOI: 10.1093/nar/gkq017
TRAP150 activates pre-mRNA splicing and promotes nuclear mRNA degradation
Abstract
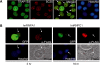
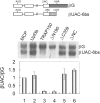
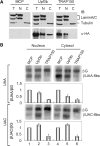
TRAP150 has been identified as a subunit of the transcription regulatory complex TRAP/Mediator, and also a component of the spliceosome. The exact function of TRAP150, however, remains unclear. We recently identified TRAP150 by its association with the mRNA export factor TAP. TRAP150 contains an arginine/serine-rich domain and has sequence similarity with the cell death-promoting transcriptional repressor BCLAF1. We found that TRAP150 co-localizes with splicing factors in nuclear speckles, and is required for pre-mRNA splicing and activates splicing in vivo. TRAP150 remains associated with the spliced mRNA after splicing, and accordingly, it interacts with the integral exon junction complex. Unexpectedly, when tethered to a precursor mRNA, TRAP150 can trigger mRNA degradation in the nucleus. However, unlike nonsense-mediated decay, TRAP150-mediated mRNA decay is irrespective of the presence of upstream stop codons and occurs in the nucleus. Moreover, TRAP150 activates pre-mRNA splicing and induces mRNA degradation by its separable functional domains. Therefore, TRAP150 represents a multi-functional protein involved in nuclear mRNA metabolism.
Figures




Similar articles
-
Btf and TRAP150 have distinct roles in regulating subcellular mRNA distribution.Nucleus. 2013 May-Jun;4(3):229-40. doi: 10.4161/nucl.25187. Epub 2013 May 29. Nucleus. 2013. PMID: 23778535 Free PMC article.
-
Alignment of Mitotic Chromosomes in Human Cells Involves SR-Like Splicing Factors Btf and TRAP150.Int J Mol Sci. 2017 Sep 12;18(9):1956. doi: 10.3390/ijms18091956. Int J Mol Sci. 2017. PMID: 28895891 Free PMC article.
-
The protein Aly links pre-messenger-RNA splicing to nuclear export in metazoans.Nature. 2000 Sep 21;407(6802):401-5. doi: 10.1038/35030160. Nature. 2000. PMID: 11014198
-
A conserved mRNA export machinery coupled to pre-mRNA splicing.Cell. 2002 Feb 22;108(4):523-31. doi: 10.1016/s0092-8674(02)00627-x. Cell. 2002. PMID: 11909523 Review.
-
Nonsense-mediated mRNA decay: splicing, translation and mRNP dynamics.Nat Rev Mol Cell Biol. 2004 Feb;5(2):89-99. doi: 10.1038/nrm1310. Nat Rev Mol Cell Biol. 2004. PMID: 15040442 Review.
Cited by
-
RBM4 Modulates Radial Migration via Alternative Splicing of Dab1 during Cortex Development.Mol Cell Biol. 2018 May 29;38(12):e00007-18. doi: 10.1128/MCB.00007-18. Print 2018 Jun 15. Mol Cell Biol. 2018. PMID: 29581187 Free PMC article.
-
Role of the DNA Damage Response in Human Papillomavirus RNA Splicing and Polyadenylation.Int J Mol Sci. 2018 Jun 12;19(6):1735. doi: 10.3390/ijms19061735. Int J Mol Sci. 2018. PMID: 29895741 Free PMC article. Review.
-
Subversion of phosphorylated SR proteins by enterovirus A71 in IRES-dependent translation revealed by RNA-interactome analysis.PLoS Pathog. 2025 Jun 16;21(6):e1013242. doi: 10.1371/journal.ppat.1013242. eCollection 2025 Jun. PLoS Pathog. 2025. PMID: 40522993 Free PMC article.
-
THRAP3 interacts with HELZ2 and plays a novel role in adipocyte differentiation.Mol Endocrinol. 2013 May;27(5):769-80. doi: 10.1210/me.2012-1332. Epub 2013 Mar 22. Mol Endocrinol. 2013. PMID: 23525231 Free PMC article.
-
Inhibition of orf virus replication in goat skin fibroblast cells by the HSPA1B protein, as demonstrated by iTRAQ-based quantitative proteome analysis.Arch Virol. 2020 Nov;165(11):2561-2587. doi: 10.1007/s00705-020-04789-y. Epub 2020 Sep 2. Arch Virol. 2020. PMID: 32876795 Free PMC article.
References
-
- Moore MJ, Proudfoot NJ. Pre-mRNA processing reaches back to transcription and ahead to translation. Cell. 2009;136:688–700. - PubMed
-
- Maniatis T, Reed R. An extensive network of coupling among gene expression machines. Nature. 2002;416:499–506. - PubMed
-
- Wahl MC, Will CL, Lührmann R. The spliceosome: design principles of a dynamic RNP machine. Cell. 2009;136:701–718. - PubMed
-
- Tange TO, Nott A, Moore MJ. The ever-increasing complexities of the exon junction complex. Curr. Opin. Cell. Biol. 2004;16:279–284. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

