Epigenetic repression of E-cadherin by human papillomavirus 16 E7 protein
- PMID: 20123756
- PMCID: PMC2864410
- DOI: 10.1093/carcin/bgq027
Epigenetic repression of E-cadherin by human papillomavirus 16 E7 protein
Abstract
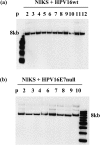
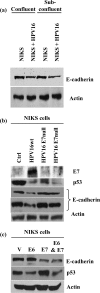
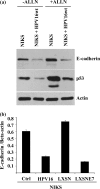
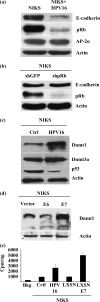
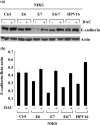
A common feature shared between several human cancer-associated viruses, such as Epstein-Barr virus, Hepatitis B virus and Hepatitis C virus, and Human papillomavirus (HPV) is the ability to reduce the expression of cellular E-cadherin. Since E-cadherin is used by Langerhans cells to move through the stratified epithelium, its reduction may affect the efficiency by which the immune system responds to HPV infection and the length of persistent HPV infections. We observed that the E7 protein of this virus (HPV16) is most efficient at reducing E-cadherin levels. This E7 activity is independent of retinoblastoma protein or AP-2alpha degradation. Instead it is associated with augmentation of cellular DNA methyltransferase I (Dnmt1) activity. Significantly, inhibition of Dnmt activity re-established E-cadherin levels of the cells, presenting the possibility that similar epigenetic intervention clinically may be a way to re-establish the influx of Langerhans cells into infected epithelium to counteract HPV persistence.
Figures

Similar articles
-
CDH1 and SNAI1 are regulated by E7 from human papillomavirus types 16 and 18.Int J Oncol. 2020 Jul;57(1):301-313. doi: 10.3892/ijo.2020.5039. Epub 2020 Apr 6. Int J Oncol. 2020. PMID: 32319591
-
Silencing of E7 oncogene restores functional E-cadherin expression in human papillomavirus 16-transformed keratinocytes.Carcinogenesis. 2008 Jul;29(7):1441-7. doi: 10.1093/carcin/bgn145. Epub 2008 Jun 19. Carcinogenesis. 2008. PMID: 18566017
-
HPV-16 E6/E7 promotes cell migration and invasion in cervical cancer via regulating cadherin switch in vitro and in vivo.Arch Gynecol Obstet. 2015 Dec;292(6):1345-54. doi: 10.1007/s00404-015-3787-x. Epub 2015 Jun 21. Arch Gynecol Obstet. 2015. PMID: 26093522
-
Human Papillomavirus E7 Oncoprotein Subverts Host Innate Immunity via SUV39H1-Mediated Epigenetic Silencing of Immune Sensor Genes.J Virol. 2020 Jan 31;94(4):e01812-19. doi: 10.1128/JVI.01812-19. Print 2020 Jan 31. J Virol. 2020. PMID: 31776268 Free PMC article.
-
Papillomavirus E6 and E7 proteins and their cellular targets.Front Biosci. 2008 Jan 1;13:1003-17. doi: 10.2741/2739. Front Biosci. 2008. PMID: 17981607 Review.
Cited by
-
Protein expression and promoter methylation of the candidate biomarker TCF21 in head and neck squamous cell carcinoma.Cell Oncol (Dordr). 2013 Jun;36(3):213-24. doi: 10.1007/s13402-013-0129-5. Epub 2013 Mar 26. Cell Oncol (Dordr). 2013. PMID: 23529585
-
Plant-Derived Epi-Nutraceuticals as Potential Broad-Spectrum Anti-Viral Agents.Nutrients. 2023 Nov 8;15(22):4719. doi: 10.3390/nu15224719. Nutrients. 2023. PMID: 38004113 Free PMC article. Review.
-
The Hallmarks of Cervical Cancer: Molecular Mechanisms Induced by Human Papillomavirus.Biology (Basel). 2024 Jan 27;13(2):77. doi: 10.3390/biology13020077. Biology (Basel). 2024. PMID: 38392296 Free PMC article. Review.
-
Nip the HPV encoded evil in the cancer bud: HPV reshapes TRAILs and signaling landscapes.Cancer Cell Int. 2013 Jun 17;13(1):61. doi: 10.1186/1475-2867-13-61. Cancer Cell Int. 2013. PMID: 23773282 Free PMC article.
-
Human papillomavirus type 16 E7 oncoprotein upregulates the retinoic acid receptor-beta expression in cervical cancer cell lines and K14E7 transgenic mice.Mol Cell Biochem. 2015 Oct;408(1-2):261-72. doi: 10.1007/s11010-015-2504-1. Epub 2015 Jul 15. Mol Cell Biochem. 2015. PMID: 26173416
References
-
- Howley PM. The role of papillomaviruses in human cancer. Important Adv. Oncol. 1987:55–73. - PubMed
-
- zur Hausen H, et al. Viruses in the etiology of human genital cancer. Prog. Med. Virol. 1984;30:170–186. - PubMed
-
- Huibregtse JM, et al. E6-AP directs the HPV E6-dependent inactivation of p53 and is representative of a family of structurally and functionally related proteins. Cold Spring Harb. Symp. Quant. Biol. 1994;59:237–245. - PubMed
-
- Scheffner M, et al. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990;63:1129–1136. - PubMed

