Acute exposure to ozone exacerbates acetaminophen-induced liver injury in mice
- PMID: 20123758
- PMCID: PMC2855356
- DOI: 10.1093/toxsci/kfq034
Acute exposure to ozone exacerbates acetaminophen-induced liver injury in mice
Abstract
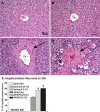
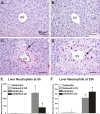
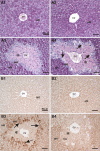
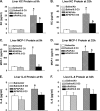
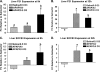
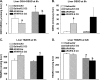
Ozone (O(3)), an oxidant air pollutant in photochemical smog, principally targets epithelial cells lining the respiratory tract. However, changes in gene expression have also been reported in livers of O(3)-exposed mice. The principal aim of the present study was to determine if acute exposure to environmentally relevant concentrations of O(3) could cause exacerbation of drug-induced liver injury in mice. Overdose with acetaminophen (APAP) is the most common cause of drug-induced liver injury in developed countries. In the present study, we examined the hepatic effects of acute O(3) exposure in mice pretreated with a hepatotoxic dose of APAP. C57BL/6 male mice were fasted overnight and then given APAP (300 mg/kg ip) or saline vehicle (0 mg/kg APAP). Two hours later, mice were exposed to 0, 0.25, or 0.5 ppm O(3) for 6 h and then sacrificed 9 or 32 h after APAP administration (1 or 24 h after O(3) exposure, respectively). Animals euthanized at 32 h were given 5-bromo-2-deoxyuridine 2 h before sacrifice to identify hepatocytes undergoing reparative DNA synthesis. Saline-treated mice exposed to either air or O(3) had no liver injury. All APAP-treated mice developed marked centrilobular hepatocellular necrosis that increased in severity with time after APAP exposure. O(3) exposure increased the severity of APAP-induced liver injury as indicated by an increase in necrotic hepatic tissue and plasma alanine aminotransferase activity. O(3) also caused an increase in neutrophil accumulation in livers of APAP-treated animals. APAP induced a 10-fold increase in the number of bromodeoxyuridine-labeled hepatocytes that was markedly attenuated by O(3) exposure. Gene expression analysis 9 h after APAP revealed differential expression of genes involved in inflammation, oxidative stress, and cellular regeneration in mice treated with APAP and O(3) compared to APAP or O(3) alone, providing some indications of the mechanisms behind the APAP and O(3) potentiation. These results suggest that acute exposure to near ambient concentrations of this oxidant air pollutant may exacerbate drug-induced liver injury by delaying hepatic repair.
Figures




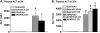




References
-
- Amimoto T, Matsura T, Koyama SY, Nakanishi T, Yamada K, Kajiyama G. Acetaminophen-induced hepatic injury in mice: the role of lipid peroxidation and effects of pretreatment with coenzyme Q10 and alpha-tocopherol. Free Radic. Biol. Med. 1995;19:169–176. - PubMed
-
- Bornholdt J, Dybdahl M, Vogel U, Hansen M, Loft S, Wallin H. Inhalation of ozone induces DNA strand breaks and inflammation in mice. Mutat. Res. 2002;520:63–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

