Type I IFN controls chikungunya virus via its action on nonhematopoietic cells
- PMID: 20123960
- PMCID: PMC2822618
- DOI: 10.1084/jem.20090851
Type I IFN controls chikungunya virus via its action on nonhematopoietic cells
Abstract
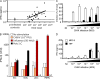
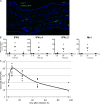
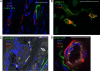
Chikungunya virus (CHIKV) is the causative agent of an outbreak that began in La Réunion in 2005 and remains a major public health concern in India, Southeast Asia, and southern Europe. CHIKV is transmitted to humans by mosquitoes and the associated disease is characterized by fever, myalgia, arthralgia, and rash. As viral load in infected patients declines before the appearance of neutralizing antibodies, we studied the role of type I interferon (IFN) in CHIKV pathogenesis. Based on human studies and mouse experimentation, we show that CHIKV does not directly stimulate type I IFN production in immune cells. Instead, infected nonhematopoietic cells sense viral RNA in a Cardif-dependent manner and participate in the control of infection through their production of type I IFNs. Although the Cardif signaling pathway contributes to the immune response, we also find evidence for a MyD88-dependent sensor that is critical for preventing viral dissemination. Moreover, we demonstrate that IFN-alpha/beta receptor (IFNAR) expression is required in the periphery but not on immune cells, as IFNAR(-/-)-->WT bone marrow chimeras are capable of clearing the infection, whereas WT-->IFNAR(-/-) chimeras succumb. This study defines an essential role for type I IFN, produced via cooperation between multiple host sensors and acting directly on nonhematopoietic cells, in the control of CHIKV.
Figures

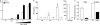



References
-
- Aricò E., Robertson K.A., Belardelli F., Ferrantini M., Nash A.A. 2004. Vaccination with inactivated murine gammaherpesvirus 68 strongly limits viral replication and latency and protects type I IFN receptor knockout mice from a lethal infection. Vaccine. 22:1433–1440 10.1016/j.vaccine.2003.10.015 - DOI - PubMed
-
- Barry G., Breakwell L., Fragkoudis R., Attarzadeh-Yazdi G., Rodriguez-Andres J., Kohl A., Fazakerley J.K. 2009. PKR acts early in infection to suppress Semliki Forest virus production and strongly enhances the type I interferon response. J. Gen. Virol. 90:1382–1391 10.1099/vir.0.007336-0 - DOI - PMC - PubMed
-
- Bieback K., Lien E., Klagge I.M., Avota E., Schneider-Schaulies J., Duprex W.P., Wagner H., Kirschning C.J., Ter Meulen V., Schneider-Schaulies S. 2002. Hemagglutinin protein of wild-type measles virus activates toll-like receptor 2 signaling. J. Virol. 76:8729–8736 10.1128/JVI.76.17.8729-8736.2002 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

