Lipid phosphate phosphatase 3 stabilization of beta-catenin induces endothelial cell migration and formation of branching point structures
- PMID: 20123964
- PMCID: PMC2838065
- DOI: 10.1128/MCB.00038-09
Lipid phosphate phosphatase 3 stabilization of beta-catenin induces endothelial cell migration and formation of branching point structures
Abstract
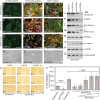
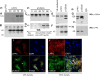
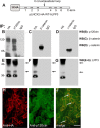
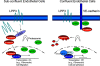
Endothelial cell (EC) migration, cell-cell adhesion, and the formation of branching point structures are considered hallmarks of angiogenesis; however, the underlying mechanisms of these processes are not well understood. Lipid phosphate phosphatase 3 (LPP3) is a recently described p120-catenin-associated integrin ligand localized in adherens junctions (AJs) of ECs. Here, we tested the hypothesis that LPP3 stimulates beta-catenin/lymphoid enhancer binding factor 1 (beta-catenin/LEF-1) to induce EC migration and formation of branching point structures. In subconfluent ECs, LPP3 induced expression of fibronectin via beta-catenin/LEF-1 signaling in a phosphatase and tensin homologue (PTEN)-dependent manner. In confluent ECs, depletion of p120-catenin restored LPP3-mediated beta-catenin/LEF-1 signaling. Depletion of LPP3 resulted in destabilization of beta-catenin, which in turn reduced fibronectin synthesis and deposition, which resulted in inhibition of EC migration. Accordingly, reexpression of beta-catenin but not p120-catenin in LPP3-depleted ECs restored de novo synthesis of fibronectin, which mediated EC migration and formation of branching point structures. In confluent ECs, however, a fraction of p120-catenin associated and colocalized with LPP3 at the plasma membrane, via the C-terminal cytoplasmic domain, thereby limiting the ability of LPP3 to stimulate beta-catenin/LEF-1 signaling. Thus, our study identified a key role for LPP3 in orchestrating PTEN-mediated beta-catenin/LEF-1 signaling in EC migration, cell-cell adhesion, and formation of branching point structures.
Figures







Similar articles
-
Endothelial lipid phosphate phosphatase-3 deficiency that disrupts the endothelial barrier function is a modifier of cardiovascular development.Cardiovasc Res. 2016 Jul 1;111(1):105-18. doi: 10.1093/cvr/cvw090. Epub 2016 Apr 28. Cardiovasc Res. 2016. PMID: 27125875 Free PMC article.
-
Role of lipid phosphate phosphatase 3 in human aortic endothelial cell function.Cardiovasc Res. 2016 Dec;112(3):702-713. doi: 10.1093/cvr/cvw217. Epub 2016 Sep 30. Cardiovasc Res. 2016. PMID: 27694435 Free PMC article.
-
Lipid phosphate phosphatase-3 regulates tumor growth via β-catenin and CYCLIN-D1 signaling.Mol Cancer. 2011 May 11;10:51. doi: 10.1186/1476-4598-10-51. Mol Cancer. 2011. PMID: 21569306 Free PMC article.
-
p120-Catenin: a novel regulator of innate immunity and inflammation.Crit Rev Immunol. 2012;32(2):127-38. doi: 10.1615/critrevimmunol.v32.i2.20. Crit Rev Immunol. 2012. PMID: 23216611 Free PMC article. Review.
-
Lipid phosphate phosphatases and lipid phosphate signalling.Biochem Soc Trans. 2005 Dec;33(Pt 6):1370-4. doi: 10.1042/BST0331370. Biochem Soc Trans. 2005. PMID: 16246121 Review.
Cited by
-
Lipid phosphate phosphatases and their roles in mammalian physiology and pathology.J Lipid Res. 2015 Nov;56(11):2048-60. doi: 10.1194/jlr.R058362. Epub 2015 Mar 26. J Lipid Res. 2015. PMID: 25814022 Free PMC article. Review.
-
A requirement for Krüppel Like Factor-4 in the maintenance of endothelial cell quiescence.Front Cell Dev Biol. 2022 Nov 8;10:1003028. doi: 10.3389/fcell.2022.1003028. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36425528 Free PMC article.
-
Glypican-4 regulated actin cytoskeletal reorganization in glucocorticoid treated trabecular meshwork cells and involvement of Wnt/PCP signaling.J Cell Physiol. 2023 Mar;238(3):631-646. doi: 10.1002/jcp.30953. Epub 2023 Feb 2. J Cell Physiol. 2023. PMID: 36727620 Free PMC article.
-
Lipid-induced epigenomic changes in human macrophages identify a coronary artery disease-associated variant that regulates PPAP2B Expression through Altered C/EBP-beta binding.PLoS Genet. 2015 Apr 2;11(4):e1005061. doi: 10.1371/journal.pgen.1005061. eCollection 2015 Apr. PLoS Genet. 2015. PMID: 25835000 Free PMC article.
-
Chromatin-modifying agents convert fibroblasts to OCT4+ and VEGFR-2+ capillary tube-forming cells.PLoS One. 2017 May 3;12(5):e0176496. doi: 10.1371/journal.pone.0176496. eCollection 2017. PLoS One. 2017. PMID: 28467484 Free PMC article.
References
-
- Adams, J. C., and F. M. Watt. 1993. Regulation of development and differentiation by the extracellular matrix. Development 117:1183-1198. - PubMed
-
- Bazzoni, G., and E. Dejana. 2004. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol. Rev. 84:869-901. - PubMed
-
- Bohnsack, B. L., and K. K. Hirschi. 2004. Red light, green light: signals that control endothelial cell proliferation during embryonic vascular development. Cell Cycle 3:1506-1511. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
