Serum response factor regulates hippocampal lamination and dendrite development and is connected with reelin signaling
- PMID: 20123976
- PMCID: PMC2838085
- DOI: 10.1128/MCB.01434-09
Serum response factor regulates hippocampal lamination and dendrite development and is connected with reelin signaling
Abstract
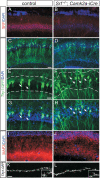
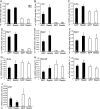
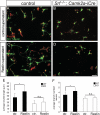
During brain development, neurons and their nerve fibers are often segregated in specific layers. The hippocampus is a well-suited model system to study lamination in health and aberrant cell/fiber lamination associated with neurological disorders. SRF (serum response factor), a transcription factor, regulates synaptic-activity-induced immediate-early gene (IEG) induction and cytoskeleton-based neuronal motility. Using early postnatal conditional SRF ablation, we uncovered distorted hippocampal lamination, including malpositioning of granule cell neurons and disruption of layer-restricted termination of commissural-associational and mossy fiber axons. Besides axons, dendrite branching and spine morphogenesis in Srf mutants were impaired, offering a first morphological basis for SRF's reported role in learning and memory. Srf mutants resemble mice lacking components of the reelin signaling cascade, a fundamental signaling entity in brain lamination. Our data indicate that reelin signaling and SRF-mediated gene transcription might be connected: reelin induces IEG and cytoskeletal genes in an SRF-dependent manner. Further, reelin-induced neurite motility is blocked in Srf mutants and constitutively active SRF rescues impaired neurite extension in reeler mouse mutants in vitro. In sum, data provided in this report show that SRF contributes to hippocampal layer and nerve fiber organization and point at a link between Srf gene transcription and reelin signaling.
Figures




Similar articles
-
Reelin signaling facilitates maturation of CA1 glutamatergic synapses.J Neurophysiol. 2007 Mar;97(3):2312-21. doi: 10.1152/jn.00869.2006. Epub 2007 Jan 17. J Neurophysiol. 2007. PMID: 17229826
-
Reelin promotes hippocampal dendrite development through the VLDLR/ApoER2-Dab1 pathway.Neuron. 2004 Jan 8;41(1):71-84. doi: 10.1016/s0896-6273(03)00819-5. Neuron. 2004. PMID: 14715136
-
Reelin Affects Signaling Pathways of a Group of Inhibitory Neurons and the Development of Inhibitory Synapses in Primary Neurons.Int J Mol Sci. 2021 Jul 13;22(14):7510. doi: 10.3390/ijms22147510. Int J Mol Sci. 2021. PMID: 34299127 Free PMC article.
-
Role of Reelin in the development and maintenance of cortical lamination.J Neural Transm (Vienna). 2009 Nov;116(11):1451-5. doi: 10.1007/s00702-009-0228-7. Epub 2009 Apr 25. J Neural Transm (Vienna). 2009. PMID: 19396394 Review.
-
Role for Reelin in stabilizing cortical architecture.Trends Neurosci. 2010 Sep;33(9):407-14. doi: 10.1016/j.tins.2010.06.001. Epub 2010 Jul 1. Trends Neurosci. 2010. PMID: 20598379 Review.
Cited by
-
Reelin induces Erk1/2 signaling in cortical neurons through a non-canonical pathway.J Biol Chem. 2014 Jul 18;289(29):20307-17. doi: 10.1074/jbc.M114.576249. Epub 2014 May 29. J Biol Chem. 2014. PMID: 24876378 Free PMC article.
-
SRF Is Required for Maintenance of Astrocytes in Non-Reactive State in the Mammalian Brain.eNeuro. 2021 Jan 29;8(1):ENEURO.0447-19.2020. doi: 10.1523/ENEURO.0447-19.2020. Print 2021 Jan-Feb. eNeuro. 2021. PMID: 33441399 Free PMC article.
-
Loss of serum response factor in mature neurons in the dentate gyrus alters the morphology of dendritic spines and hippocampus-dependent behavioral tasks.Brain Struct Funct. 2019 Nov;224(8):2691-2701. doi: 10.1007/s00429-019-01925-6. Epub 2019 Aug 2. Brain Struct Funct. 2019. PMID: 31375980 Free PMC article.
-
Synaptic localisation of SRF coactivators, MKL1 and MKL2, and their role in dendritic spine morphology.Sci Rep. 2018 Jan 15;8(1):727. doi: 10.1038/s41598-017-18905-7. Sci Rep. 2018. PMID: 29335431 Free PMC article.
-
Transcription Mapping of Embryonic Rat Brain Reveals EGR-1 Induction in SOX2 Neural Progenitor Cells.Front Mol Neurosci. 2011 May 17;4:6. doi: 10.3389/fnmol.2011.00006. eCollection 2011. Front Mol Neurosci. 2011. PMID: 21629823 Free PMC article.
References
-
- Alberti, S., S. M. Krause, O. Kretz, U. Philippar, T. Lemberger, E. Casanova, F. F. Wiebel, H. Schwarz, M. Frotscher, G. Schutz, and A. Nordheim. 2005. Neuronal migration in the murine rostral migratory stream requires serum response factor. Proc. Natl. Acad. Sci. U. S. A. 102:6148-6153. - PMC - PubMed
-
- Borrell, V., J. A. Del Río, S. Alcantara, M. Derer, A. Martinez, G. D'Arcangelo, K. Nakajima, K. Mikoshiba, P. Derer, T. Curran, and E. Soriano. 1999. Reelin regulates the development and synaptogenesis of the layer-specific entorhino-hippocampal connections. J. Neurosci. 19:1345-1358. - PMC - PubMed
-
- Borrell, V., L. Pujadas, S. Simó, D. Dura, M. Sole, J. A. Cooper, J. A. Del Río, and E. Soriano. 2007. Reelin and mDab1 regulate the development of hippocampal connections. Mol. Cell. Neurosci. 36:158-173. - PubMed
-
- Borrell, V., M. Ruiz, J. A. Del Río, and E. Soriano. 1999. Development of commissural connections in the hippocampus of reeler mice: evidence of an inhibitory influence of Cajal-Retzius cells. Exp. Neurol. 156:268-282. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
