Dysautonomia due to reduced cholinergic neurotransmission causes cardiac remodeling and heart failure
- PMID: 20123977
- PMCID: PMC2838086
- DOI: 10.1128/MCB.00996-09
Dysautonomia due to reduced cholinergic neurotransmission causes cardiac remodeling and heart failure
Abstract
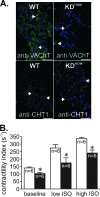
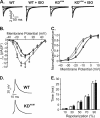
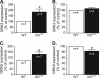
Overwhelming evidence supports the importance of the sympathetic nervous system in heart failure. In contrast, much less is known about the role of failing cholinergic neurotransmission in cardiac disease. By using a unique genetically modified mouse line with reduced expression of the vesicular acetylcholine transporter (VAChT) and consequently decreased release of acetylcholine, we investigated the consequences of altered cholinergic tone for cardiac function. M-mode echocardiography, hemodynamic experiments, analysis of isolated perfused hearts, and measurements of cardiomyocyte contraction indicated that VAChT mutant mice have decreased left ventricle function associated with altered calcium handling. Gene expression was analyzed by quantitative reverse transcriptase PCR and Western blotting, and the results indicated that VAChT mutant mice have profound cardiac remodeling and reactivation of the fetal gene program. This phenotype was attributable to reduced cholinergic tone, since administration of the cholinesterase inhibitor pyridostigmine for 2 weeks reversed the cardiac phenotype in mutant mice. Our findings provide direct evidence that decreased cholinergic neurotransmission and underlying autonomic imbalance cause plastic alterations that contribute to heart dysfunction.
Figures


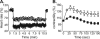

References
-
- Amorim, D. S., H. J. Dargie, K. Heer, M. Brown, D. Jenner, E. G. Olsen, P. Richardson, and J. F. Goodwin. 1981. Is there autonomic impairment in congestive (dilated) cardiomyopathy? Lancet i:525-527. - PubMed
-
- Bartholomeu, J. B., A. S. Vanzelli, N. P. Rolim, J. C. Ferreira, L. R. Bechara, L. Y. Tanaka, K. T. Rosa, M. M. Alves, A. Medeiros, K. C. Mattos, M. A. Coelho, M. C. Irigoyen, E. M. Krieger, J. E. Krieger, C. E. Negrao, P. R. Ramires, S. Guatimosim, and P. C. Brum. 2008. Intracellular mechanisms of specific beta-adrenoceptor antagonists involved in improved cardiac function and survival in a genetic model of heart failure. J. Mol. Cell. Cardiol. 45:240-249. - PubMed
-
- Bers, D. M. 2006. Altered cardiac myocyte Ca regulation in heart failure. Physiology (Bethesda) 21:380-387. - PubMed
-
- Bers, D. M. 2002. Cardiac excitation-contraction coupling. Nature 415:198-205. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
