Establishment of extracellular signal-regulated kinase 1/2 bistability and sustained activation through Sprouty 2 and its relevance for epithelial function
- PMID: 20123980
- PMCID: PMC2838067
- DOI: 10.1128/MCB.01003-09
Establishment of extracellular signal-regulated kinase 1/2 bistability and sustained activation through Sprouty 2 and its relevance for epithelial function
Abstract
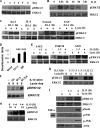
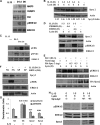
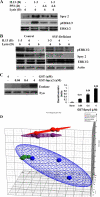
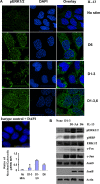
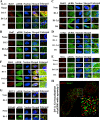
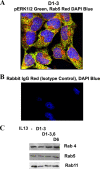
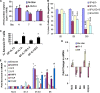
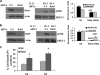
Our objective was to establish an experimental model of a self-sustained and bistable extracellular signal-regulated kinase 1/2 (ERK1/2) signaling process. A single stimulation of cells with cytokines causes rapid ERK1/2 activation, which returns to baseline in 4 h. Repeated stimulation leads to sustained activation of ERK1/2 but not Jun N-terminal protein kinase (JNK), p38, or STAT6. The ERK1/2 activation lasts for 3 to 7 days and depends upon a positive-feedback mechanism involving Sprouty 2. Overexpression of Sprouty 2 induces, and its genetic deletion abrogates, ERK1/2 bistability. Sprouty 2 directly activates Fyn kinase, which then induces ERK1/2 activation. A genome-wide microarray analysis shows that the bistable phospho-ERK1/2 (pERK1/2) does not induce a high level of gene transcription. This is due to its nuclear exclusion and compartmentalization to Rab5+ endosomes. Cells with sustained endosomal pERK1/2 manifest resistance against growth factor withdrawal-induced cell death. They are primed for heightened cytokine production. Epithelial cells from cases of human asthma and from a mouse model of chronic asthma manifest increased pERK1/2, which is associated with Rab5+ endosomes. The increase in pERK1/2 was associated with a simultaneous increase in Sprouty 2 expression in these tissues. Thus, we have developed a cellular model of sustained ERK1/2 activation, which may provide a mechanistic understanding of self-sustained biological processes in chronic illnesses such as asthma.
Figures

Similar articles
-
hCG activates Epac-Erk1/2 signaling regulating Progesterone Receptor expression and function in human endometrial stromal cells.Mol Hum Reprod. 2017 Jun 1;23(6):393-405. doi: 10.1093/molehr/gax015. Mol Hum Reprod. 2017. PMID: 28333280
-
Differential phosphorylation of the phosphoinositide 3-phosphatase MTMR2 regulates its association with early endosomal subtypes.J Cell Sci. 2013 Mar 15;126(Pt 6):1333-44. doi: 10.1242/jcs.113928. Epub 2013 Feb 1. J Cell Sci. 2013. PMID: 23378027
-
Grape seed extract inhibits EGF-induced and constitutively active mitogenic signaling but activates JNK in human prostate carcinoma DU145 cells: possible role in antiproliferation and apoptosis.Oncogene. 2003 Mar 6;22(9):1302-16. doi: 10.1038/sj.onc.1206265. Oncogene. 2003. PMID: 12618755
-
Mitogen-activated protein kinase signalling and ERK1/2 bistability in asthma.Clin Exp Allergy. 2011 Feb;41(2):149-59. doi: 10.1111/j.1365-2222.2010.03658.x. Epub 2010 Dec 1. Clin Exp Allergy. 2011. PMID: 21121982 Free PMC article. Review.
-
The developing story of Sprouty and cancer.Cancer Metastasis Rev. 2014 Sep;33(2-3):695-720. doi: 10.1007/s10555-014-9497-1. Cancer Metastasis Rev. 2014. PMID: 24744103 Free PMC article. Review.
Cited by
-
Vinculin and Rab5 complex is required [correction of requited]for uptake of Staphylococcus aureus and interleukin-6 expression.PLoS One. 2014 Jan 23;9(1):e87373. doi: 10.1371/journal.pone.0087373. eCollection 2014. PLoS One. 2014. PMID: 24466349 Free PMC article.
-
Mechanism of TH2/TH17-predominant and neutrophilic TH2/TH17-low subtypes of asthma.J Allergy Clin Immunol. 2017 May;139(5):1548-1558.e4. doi: 10.1016/j.jaci.2016.08.032. Epub 2016 Oct 1. J Allergy Clin Immunol. 2017. PMID: 27702673 Free PMC article.
-
Effects of β-blockers on house dust mite-driven murine models pre- and post-development of an asthma phenotype.Pulm Pharmacol Ther. 2017 Oct;46:30-40. doi: 10.1016/j.pupt.2017.07.004. Epub 2017 Jul 17. Pulm Pharmacol Ther. 2017. PMID: 28729042 Free PMC article.
-
Persistence of asthma requires multiple feedback circuits involving type 2 innate lymphoid cells and IL-33.J Allergy Clin Immunol. 2015 Jul;136(1):59-68.e14. doi: 10.1016/j.jaci.2014.11.037. Epub 2015 Jan 21. J Allergy Clin Immunol. 2015. PMID: 25617223 Free PMC article.
-
An unbiased tissue transcriptome analysis identifies potential markers for skin phenotypes and therapeutic responses in atopic dermatitis.Nat Commun. 2025 Jun 2;16(1):4981. doi: 10.1038/s41467-025-59340-x. Nat Commun. 2025. PMID: 40456762 Free PMC article.
References
-
- Bashor, C. J., N. C. Helman, S. Yan, and W. A. Lim. 2008. Using engineered scaffold interactions to reshape MAP kinase pathway signaling dynamics. Science 319:1539-1543. - PubMed
-
- Casci, T., J. Vinós, and M. Freeman. 1999. Sprouty, an intracellular inhibitor of Ras signaling. Cell 96:655-665. - PubMed
-
- Chandramouli, S., C. Y. Yu, P. Yusoff, D. H. Lao, H. F. Leong, K. Mizuno, and G. R. Guy. 2008. Tesk1 interacts with Spry2 to abrogate its inhibition of ERK phosphorylation downstream of receptor tyrosine kinase signaling. J. Biol. Chem. 283:1679-1691. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
