Maspin (SERPINB5) is an obligate intracellular serpin
- PMID: 20123984
- PMCID: PMC2856292
- DOI: 10.1074/jbc.M109.073171
Maspin (SERPINB5) is an obligate intracellular serpin
Abstract
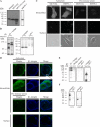
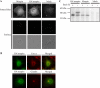
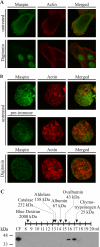
Maspin (SERPINB5) is a tumor suppressor lost in breast and prostate cancer whose molecular function is unknown. It is a non-inhibitory member of the clade B serpins suggested to play a role in a plethora of intracellular and extracellular settings, yet its normal cellular distribution has never been clarified. Here we investigate the distribution of maspin in non-transformed human epithelial cells. By indirect immunofluorescence, maspin has a nucleocytoplasmic distribution in breast (MCF10A) and prostate (RWPE-1) cells and, by immunoblotting and pulse-chase analyses, is neither glycosylated nor secreted. Cell surface biotinylation studies also show that maspin is not present at the cell surface. Differentiation of MCF10A cells into three-dimensional acini results in the redistribution of maspin from the nucleus to the cytoplasm but does not result in secretion. Addition of an efficient conventional signal peptide to maspin directs it into the secretory pathway and results in glycosylation but not secretion. We further show that maspin in the cytoplasm of MCF10A cells is a soluble monomeric protein that is not detectably associated with the cytoskeleton or other extractable components. Taken together, these results suggest that maspin is restricted to an intracellular, possibly nuclear, role in which it influences cell-matrix interactions indirectly. It is probably released only as a consequence of cell damage or necrosis.
Figures


Similar articles
-
Maspin is an intracellular serpin that partitions into secretory vesicles and is present at the cell surface.J Histochem Cytochem. 1997 Dec;45(12):1697-706. doi: 10.1177/002215549704501213. J Histochem Cytochem. 1997. PMID: 9389773
-
High expression of maspin is associated with early tumor relapse in breast cancer.Hum Pathol. 2009 Aug;40(8):1143-51. doi: 10.1016/j.humpath.2009.02.006. Epub 2009 May 8. Hum Pathol. 2009. PMID: 19427667
-
Maspin plays an essential role in early embryonic development.Development. 2004 Apr;131(7):1479-89. doi: 10.1242/dev.01048. Epub 2004 Feb 25. Development. 2004. PMID: 14985257
-
An emerging role for the nuclear localization of maspin in the suppression of tumor progression and metastasis.Biochem Cell Biol. 2012 Feb;90(1):22-38. doi: 10.1139/o11-053. Epub 2011 Nov 2. Biochem Cell Biol. 2012. PMID: 22047058 Review.
-
The Opportunity of Precision Medicine for Breast Cancer With Context-Sensitive Tumor Suppressor Maspin.J Cell Biochem. 2017 Jul;118(7):1639-1647. doi: 10.1002/jcb.25969. Epub 2017 Mar 21. J Cell Biochem. 2017. PMID: 28262971 Free PMC article. Review.
Cited by
-
Identification of an intrinsic determinant critical for maspin subcellular localization and function.PLoS One. 2013 Nov 21;8(11):e74502. doi: 10.1371/journal.pone.0074502. eCollection 2013. PLoS One. 2013. PMID: 24278104 Free PMC article.
-
Proteolytic histone modification by mast cell tryptase, a serglycin proteoglycan-dependent secretory granule protease.J Biol Chem. 2014 Mar 14;289(11):7682-90. doi: 10.1074/jbc.M113.546895. Epub 2014 Jan 29. J Biol Chem. 2014. PMID: 24478313 Free PMC article.
-
Inhibition of the SERPINB5/HSP90AA1 axis restrains the proliferation and invasion of rectal cancer.World J Gastroenterol. 2025 Mar 21;31(11):103412. doi: 10.3748/wjg.v31.i11.103412. World J Gastroenterol. 2025. PMID: 40124262 Free PMC article.
-
Maspin, the molecular bridge between the plasminogen activator system and beta1 integrin that facilitates cell adhesion.J Biol Chem. 2011 Jul 15;286(28):24599-607. doi: 10.1074/jbc.M111.235788. Epub 2011 May 23. J Biol Chem. 2011. PMID: 21606500 Free PMC article.
-
Maspin/SerpinB5 is a cytoskeleton-binding protein that regulates epithelial cell shape.Commun Biol. 2025 Aug 22;8(1):1262. doi: 10.1038/s42003-025-08688-3. Commun Biol. 2025. PMID: 40846899 Free PMC article.
References
-
- Zou Z., Anisowicz A., Hendrix M. J., Thor A., Neveu M., Sheng S., Rafidi K., Seftor E., Sager R. (1994) Science 263, 526–529 - PubMed
-
- Gao F., Shi H. Y., Daughty C., Cella N., Zhang M. (2004) Development 131, 1479–1489 - PubMed
-
- Ngamkitidechakul C., Warejcka D. J., Burke J. M., O'Brien W. J., Twining S. S. (2003) J. Biol. Chem. 278, 31796–31806 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

