Conformation change in a self-recognizing autotransporter modulates bacterial cell-cell interaction
- PMID: 20123991
- PMCID: PMC2856270
- DOI: 10.1074/jbc.M109.069070
Conformation change in a self-recognizing autotransporter modulates bacterial cell-cell interaction
Abstract
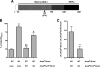
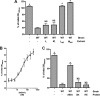
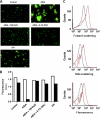
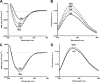
Bacteria mostly live as multicellular communities, although they are unicellular organisms, yet the mechanisms that tie individual bacteria together are often poorly understood. The adhesin involved in diffuse adherence (AIDA-I) is an adhesin of diarrheagenic Escherichia coli strains. AIDA-I also mediates bacterial auto-aggregation and biofilm formation and thus could be important for the organization of communities of pathogens. Using purified protein and whole bacteria, we provide direct evidence that AIDA-I promotes auto-aggregation by interacting with itself. Using various biophysical and biochemical techniques, we observed a conformational change in the protein during AIDA-AIDA interactions, strengthening the notion that this is a highly specific interaction. The self-association of AIDA-I is of high affinity but can be modulated by sodium chloride. We observe that a bile salt, sodium deoxycholate, also prevents AIDA-I oligomerization and bacterial auto-aggregation. Thus, we propose that AIDA-I, and most likely other similar autotransporters such as antigen 43 (Ag43) and TibA, organize bacterial communities of pathogens through a self-recognition mechanism that is sensitive to the environment. This could permit bacteria to switch between multicellular and unicellular lifestyles to complete their infection.
Figures
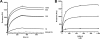

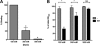
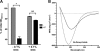
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

