Transcriptional regulation of chitin synthases by calcineurin controls paradoxical growth of Aspergillus fumigatus in response to caspofungin
- PMID: 20124000
- PMCID: PMC2849361
- DOI: 10.1128/AAC.00854-09
Transcriptional regulation of chitin synthases by calcineurin controls paradoxical growth of Aspergillus fumigatus in response to caspofungin
Abstract
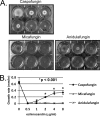
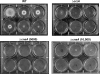
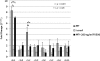
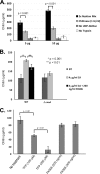
Attenuated activity of echinocandin antifungals at high concentrations, known as the "paradoxical effect," is a well-established phenomenon in Candida albicans and Aspergillus fumigatus. In the yeast C. albicans, upregulation of chitin biosynthesis via the protein kinase C (PKC), high-osmolarity glycerol response (HOG), and Ca(2+)/calcineurin signaling pathways is an important cell wall stress response that permits growth in the presence of high concentrations of echinocandins. However, nothing is known of the molecular mechanisms regulating the mold A. fumigatus and its paradoxical response to echinocandins. Here, we show that the laboratory strain of A. fumigatus and five of seven clinical A. fumigatus isolates tested display various magnitudes of paradoxical growth in response to caspofungin. Interestingly, none of the eight strains showed paradoxical growth in the presence of micafungin or anidulafungin. Treatment of the DeltacnaA and DeltacrzA strains, harboring gene deletions of the calcineurin A subunit and the calcineurin-dependent transcription factor, respectively, with high concentrations of caspofungin revealed that the A. fumigatus paradoxical effect is calcineurin pathway dependent. Exploring a molecular role for CnaA in the compensatory chitin biosynthetic response, we found that caspofungin treatment resulted in increased chitin synthase gene expression, leading to a calcineurin-dependent increase in chitin synthase activity. Taken together, our data suggest a mechanistic role for A. fumigatus calcineurin signaling in the chitin biosynthetic response observed during paradoxical growth in the presence of high-dose caspofungin treatment.
Figures

References
-
- Agarwal, A. K., P. D. Rogers, S. R. Baerson, M. R. Jacob, K. S. Barker, J. D. Cleary, L. A. Walker, D. G. Nagle, and A. M. Clark.2003. Genome-wide expression profiling of the response to polyene, pyrimidine, azole, and echinocandin antifungal agents in Saccharomyces cerevisiae. J. Biol. Chem. 278:34998-35015. - PubMed
-
- Ankarcrona, M., J. M. Dypbukt, S. Orrenius, and P. Nicotera.1996. Calcineurin and mitochondrial function in glutamate-induced neuronal cell death. FEBS Lett. 394:321-324. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

