Evaluation of a (64)Cu-labeled cystine-knot peptide based on agouti-related protein for PET of tumors expressing alphavbeta3 integrin
- PMID: 20124048
- PMCID: PMC4143171
- DOI: 10.2967/jnumed.109.069831
Evaluation of a (64)Cu-labeled cystine-knot peptide based on agouti-related protein for PET of tumors expressing alphavbeta3 integrin
Abstract
Recently, a truncated form of the agouti-related protein (AgRP), a 4-kDa cystine-knot peptide of human origin, was used as a scaffold to engineer mutants that bound to alpha(v)beta(3) integrin with high affinity and specificity. In this study, we evaluated the potential of engineered integrin-binding AgRP peptides for use as cancer imaging agents in living subjects.
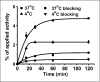
Methods: Engineered AgRP peptides were prepared by solid-phase peptide synthesis and were folded in vitro and purified by reversed-phase high-performance liquid chromatography. Competition assays were used to measure the relative binding affinities of engineered AgRP peptides for integrin receptors expressed on the surface of U87MG glioblastoma cells. The highest-affinity mutant, AgRP clone 7C, was site-specifically conjugated with 1,4,7,10-tetra-azacyclododecane-N,N',N''N'''-tetraacetic acid (DOTA). The resulting bioconjugate, DOTA-AgRP-7C, was radiolabeled with (64)Cu for biodistribution analysis and small-animal PET studies in mice bearing U87MG tumor xenografts. In addition to serum stability, the in vivo metabolic stability of (64)Cu-DOTA-AgRP-7C was assessed after injection and probe recovery from mouse kidney, liver, tumor, and urine.
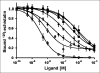
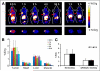
Results: AgRP-7C and DOTA-AgRP-7C bound with high affinity to integrin receptors expressed on U87MG cells (half maximal inhibitory concentration values, 20 +/- 4 and 14 +/- 2 nM, respectively). DOTA-AgRP-7C was labeled with (64)Cu with high radiochemical purity (>99%). In biodistribution and small-animal PET studies, (64)Cu-DOTA-AgRP-7C displayed rapid blood clearance, good tumor uptake and retention (2.70 +/- 0.93 percentage injected dose per gram [%ID/g] and 2.37 +/- 1.04 %ID/g at 2 and 24 h, respectively), and high tumor-to-background tissue ratios. The integrin-binding specificity of (64)Cu-DOTA-AgRP-7C was confirmed in vitro and in vivo by showing that a large molar excess of the unlabeled peptidomimetic c(RGDyK) could block probe binding and tumor uptake. Serum stability and in vivo metabolite assays demonstrated that engineered AgRP peptides are sufficiently stable for in vivo molecular imaging applications.
Conclusion: A radiolabeled version of the engineered AgRP peptide 7C showed promise as a PET agent for tumors that express the alpha(v)beta(3) integrin. Collectively, these results validate AgRP-based cystine-knot peptides for use in vivo as molecular imaging agents and provide support for the general use of AgRP as a scaffold to develop targeting peptides, and hence diagnostics, against other tumor receptors.
Figures

References
-
- Gambhir SS. Molecular imaging of cancer with positron emission tomography. Nat Rev Cancer. 2002;2:683–693. - PubMed
-
- Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–580. - PubMed
-
- Weissleder R. Molecular Imaging in Cancer. Science. 2006;312:1168–1171. - PubMed
-
- Friedman M, Stahl S. Engineered affinity proteins for tumour-targeting applications. Biotechnol Appl Biochem. 2009;53:1–29. - PubMed
-
- Ladner RC. Polypeptides from phage display. A superior source of in vivo imaging agents. Q J Nucl Med. 1999;43:119–124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
