Components of the basal lamina and dystrophin-dystroglycan complex in the neurointermediate lobe of rat pituitary gland: different localizations of beta-dystroglycan, dystrobrevins, alpha1-syntrophin, and aquaporin-4
- PMID: 20124096
- PMCID: PMC2857818
- DOI: 10.1369/jhc.2010.954768
Components of the basal lamina and dystrophin-dystroglycan complex in the neurointermediate lobe of rat pituitary gland: different localizations of beta-dystroglycan, dystrobrevins, alpha1-syntrophin, and aquaporin-4
Abstract
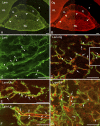
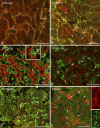
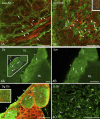
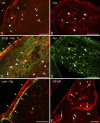
The so-called neurointermediate lobe is composed of the intermediate and neural lobes of the pituitary. The present immunohistochemical study investigated components of the basal lamina (laminin, agrin, and perlecan), the dystrophin-dystroglycan complex (dystrophin, beta-dystroglycan, alpha1-dystrobrevin, beta-dystrobrevin, utrophin, and alpha1-syntrophin), and the aquaporins (aquaporin-4 and -9). Glia markers (GFAP, S100, and glutamine synthetase) and components of connective tissue (collagen type I and fibronectin) were also labeled. In the neurohypophysis, immunostaining of basal lamina delineated meningeal invaginations. In these invaginations, vessels were seen to penetrate the organ without submerging into its parenchyma. On the parenchymal side of the invaginations, beta-dystroglycan was detected, whereas utrophin was detected in the walls of vessels. Immunostaining of alpha1-dystrobrevin and alpha1-syntrophin did not delineate the vessels. The cells of the intermediate lobe were fully immunoreactive to alpha1-dystrobrevin and alpha1-syntrophin, whereas components of the basal lamina delineated the contours of the cells. GFAP-immunoreactive processes surrounded them. Aquaporin-4 localized at the periphery of the neurohypophysis, mainly adjacent to the intermediate lobe but not along the vessels. It colocalized only partially with GFAP and not at all with alpha1-syntrophin. Aquaporin-9 was not detected. These results emphasize the possibility that the components of the dystrophin-dystroglycan complex localize differently and raise the question about the roles of dystrobrevins, alpha1-syntrophin, and aquaporin-4 in the functions of the intermediate and neural lobes, respectively.
Figures


Similar articles
-
Alterations of the perivascular dystrophin-dystroglycan complex following brain lesions: an immunohistochemical study in rats.Histol Histopathol. 2011 Nov;26(11):1435-52. doi: 10.14670/HH-26.1435. Histol Histopathol. 2011. PMID: 21938681
-
Distribution of components of basal lamina and dystrophin-dystroglycan complex in the rat pineal gland: differences from the brain tissue and between the subdivisions of the gland.Histol Histopathol. 2010 Jan;25(1):1-14. doi: 10.14670/HH-25.1. Histol Histopathol. 2010. PMID: 19924636
-
Distribution of beta-dystroglycan immunopositive globules in the subventricular zone of rat brain.Glia. 2009 Apr 15;57(6):657-66. doi: 10.1002/glia.20794. Glia. 2009. PMID: 18985737
-
Dystrophin complex functions as a scaffold for signalling proteins.Biochim Biophys Acta. 2014 Feb;1838(2):635-42. doi: 10.1016/j.bbamem.2013.08.023. Epub 2013 Sep 7. Biochim Biophys Acta. 2014. PMID: 24021238 Review.
-
The inside and out of dystroglycan post-translational modification.Neuromuscul Disord. 2012 Nov;22(11):959-65. doi: 10.1016/j.nmd.2012.05.016. Epub 2012 Jul 4. Neuromuscul Disord. 2012. PMID: 22770978 Review.
Cited by
-
Poldip2 mediates blood-brain barrier disruption and cerebral edema by inducing AQP4 polarity loss in mouse bacterial meningitis model.CNS Neurosci Ther. 2020 Dec;26(12):1288-1302. doi: 10.1111/cns.13446. Epub 2020 Aug 12. CNS Neurosci Ther. 2020. PMID: 32790044 Free PMC article.
-
Glial and perivascular structures in the subfornical organ: distinguishing the shell and core.J Histochem Cytochem. 2015 May;63(5):367-83. doi: 10.1369/0022155415575027. Epub 2015 Feb 11. J Histochem Cytochem. 2015. PMID: 25673286 Free PMC article.
-
Cellular and subcellular aquaporin-4 distribution in the mouse neurohypophysis and the effects of osmotic stimulation.J Histochem Cytochem. 2011 Jan;59(1):88-97. doi: 10.1369/jhc.2010.956805. J Histochem Cytochem. 2011. PMID: 21339176 Free PMC article.
References
-
- Adorján I, Kálmán M (2009) Distribution of β-dystroglycan immunopositive globules in the subventricular zone of rat brain. Glia 57:657–666 - PubMed
-
- Agre P, Kozono D (2003) Aquaporin water channels: molecular mechanisms for human diseases. FEBS Lett 555:72–78 - PubMed
-
- Allaerts W, Vankelecom H (2005) History and perspectives of pituitary folliculo-stellate cell research. Eur J Endocrinol 153:1–12 - PubMed
-
- Amiry-Moghaddam M, Frydenlund DS, Ottersen OP (2004) Anchoring of aquaporin-4 in brain: molecular mechanisms and implications for the physiology and pathophysiology of water transport. Neuroscience 129:999–1010 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

