A novel HLA (HLA-A*0201) transgenic rabbit model for preclinical evaluation of human CD8+ T cell epitope-based vaccines against ocular herpes
- PMID: 20124097
- PMCID: PMC3752373
- DOI: 10.4049/jimmunol.0902322
A novel HLA (HLA-A*0201) transgenic rabbit model for preclinical evaluation of human CD8+ T cell epitope-based vaccines against ocular herpes
Abstract
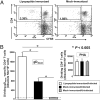
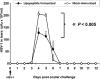
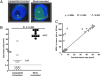
We introduced a novel humanized HLA-A*0201 transgenic (HLA Tg) rabbit model to assess the protective efficacy of a human CD8(+) T cell epitope-based vaccine against primary ocular herpes infection and disease. Each of the three immunodominant human CD8(+) T cell peptide epitopes from HSV-1 glycoprotein D (gD(53-61), gD(70-78), and gD(278-286)) were joined with a promiscuous human CD4(+) T cell peptide epitope (gD(49-82)) to construct three separate pairs of CD4-CD8 peptides. Each CD4-CD8 peptide pair was then covalently linked to an N(epsilon)-palmitoyl-lysine residue via a functional base lysine amino group to construct CD4-CD8 lipopeptides. HLA Tg rabbits were immunized s.c. with a mixture of the three CD4-CD8 HSV-1 gD lipopeptides. The HSV-gD-specific T cell responses induced by the mixture of CD4-CD8 lipopeptide vaccine and the protective efficacy against acute virus replication and ocular disease were determined. Immunization induced HSV-gD(49-82)-specific CD4(+) T cells in draining lymph node (DLN); induced HLA-restricted HSV-gD(53-61), gD(70-78), and gD(278-286)-specific CD8(+) T cells in DLN, conjunctiva, and trigeminal ganglia and reduced HSV-1 replication in tears and corneal eye disease after ocular HSV-1 challenge. In addition, the HSV-1 epitope-specific CD8(+) T cells induced in DLNs, conjunctiva, and the trigeminal ganglia were inversely proportional with corneal disease. The humanized HLA Tg rabbits appeared to be a useful preclinical animal model for investigating the immunogenicity and protective efficacy of human CD8(+) T cell epitope-based prophylactic vaccines against ocular herpes. The relevance of HLA Tg rabbits for future investigation of human CD4-CD8 epitope-based therapeutic vaccines against recurrent HSV-1 is discussed.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures





Similar articles
-
Therapeutic immunization with a mixture of herpes simplex virus 1 glycoprotein D-derived “asymptomatic” human CD8+ T-cell epitopes decreases spontaneous ocular shedding in latently infected HLA transgenic rabbits: association with low frequency of local PD-1+ TIM-3+ CD8+ exhausted T cells.J Virol. 2015 Jul;89(13):6619-32. doi: 10.1128/JVI.00788-15. J Virol. 2015. PMID: 25878105 Free PMC article.
-
A tissue-targeted prime/pull/keep therapeutic herpes simplex virus vaccine protects against recurrent ocular herpes infection and disease in HLA-A*0201 transgenic rabbits.J Virol. 2025 May 20;99(5):e0013525. doi: 10.1128/jvi.00135-25. Epub 2025 Apr 10. J Virol. 2025. PMID: 40207928 Free PMC article.
-
Human Asymptomatic Epitope Peptide/CXCL10-Based Prime/Pull Vaccine Induces Herpes Simplex Virus-Specific Gamma Interferon-Positive CD107+ CD8+ T Cells That Infiltrate the Corneas and Trigeminal Ganglia of Humanized HLA Transgenic Rabbits and Protect against Ocular Herpes Challenge.J Virol. 2018 Jul 31;92(16):e00535-18. doi: 10.1128/JVI.00535-18. Print 2018 Aug 15. J Virol. 2018. PMID: 29899087 Free PMC article.
-
Of mice and not humans: how reliable are animal models for evaluation of herpes CD8(+)-T cell-epitopes-based immunotherapeutic vaccine candidates?Vaccine. 2011 Aug 11;29(35):5824-36. doi: 10.1016/j.vaccine.2011.06.083. Epub 2011 Jun 28. Vaccine. 2011. PMID: 21718746 Free PMC article. Review.
-
Towards a rational design of an asymptomatic clinical herpes vaccine: the old, the new, and the unknown.Clin Dev Immunol. 2012;2012:187585. doi: 10.1155/2012/187585. Epub 2012 Mar 26. Clin Dev Immunol. 2012. PMID: 22548113 Free PMC article. Review.
Cited by
-
Animal Models in Eye Research: Focus on Corneal Pathologies.Int J Mol Sci. 2023 Nov 23;24(23):16661. doi: 10.3390/ijms242316661. Int J Mol Sci. 2023. PMID: 38068983 Free PMC article. Review.
-
Human Asymptomatic Epitopes Identified from the Herpes Simplex Virus Tegument Protein VP13/14 (UL47) Preferentially Recall Polyfunctional Effector Memory CD44high CD62Llow CD8+ TEM Cells and Protect Humanized HLA-A*02:01 Transgenic Mice against Ocular Herpesvirus Infection.J Virol. 2017 Jan 3;91(2):e01793-16. doi: 10.1128/JVI.01793-16. Print 2017 Jan 15. J Virol. 2017. PMID: 27847359 Free PMC article.
-
HLA-A02:01-restricted epitopes identified from the herpes simplex virus tegument protein VP11/12 preferentially recall polyfunctional effector memory CD8+ T cells from seropositive asymptomatic individuals and protect humanized HLA-A*02:01 transgenic mice against ocular herpes.J Immunol. 2015 Mar 1;194(5):2232-48. doi: 10.4049/jimmunol.1402606. Epub 2015 Jan 23. J Immunol. 2015. PMID: 25617474 Free PMC article.
-
CXCL10/CXCR3-Dependent Mobilization of Herpes Simplex Virus-Specific CD8+ TEM and CD8+ TRM Cells within Infected Tissues Allows Efficient Protection against Recurrent Herpesvirus Infection and Disease.J Virol. 2017 Jun 26;91(14):e00278-17. doi: 10.1128/JVI.00278-17. Print 2017 Jul 15. J Virol. 2017. PMID: 28468883 Free PMC article.
-
Mucosal herpes immunity and immunopathology to ocular and genital herpes simplex virus infections.Clin Dev Immunol. 2012;2012:149135. doi: 10.1155/2012/149135. Epub 2012 Dec 24. Clin Dev Immunol. 2012. PMID: 23320014 Free PMC article. Review.
References
-
- Liesegang TJ. Herpes simplex virus epidemiology and ocular importance. Cornea. 2001;20:1–13. - PubMed
-
- Banerjee K, Biswas PS, Rouse BT. Elucidating the protective and pathologic T cell species in the virus-induced corneal immunoinflammatory condition herpetic stromal keratitis. J Leukoc Biol. 2005;77:24–32. - PubMed
-
- Thomas J, Rouse BT. Immunopathogenesis of herpetic ocular disease. Immunol Res. 1997;16:375–386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

